Biogenesis and delivery of extracellular vesicles: harnessing the power of EVs for diagnostics and therapeutics
- PMID: 38234582
- PMCID: PMC10791869
- DOI: 10.3389/fmolb.2023.1330400
Biogenesis and delivery of extracellular vesicles: harnessing the power of EVs for diagnostics and therapeutics
Abstract
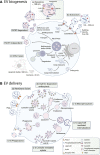
Extracellular vesicles (EVs) are membrane-enclosed particles secreted by a variety of cell types. These vesicles encapsulate a diverse range of molecules, including proteins, nucleic acids, lipids, metabolites, and even organelles derived from their parental cells. While EVs have emerged as crucial mediators of intercellular communication, they also hold immense potential as both biomarkers and therapeutic agents for numerous diseases. A thorough understanding of EV biogenesis is crucial for the development of EV-based diagnostic developments since the composition of EVs can reflect the health and disease status of the donor cell. Moreover, when EVs are taken up by target cells, they can exert profound effects on gene expression, signaling pathways, and cellular behavior, which makes these biomolecules enticing targets for therapeutic interventions. Yet, despite decades of research, the intricate processes underlying EV biogenesis by donor cells and subsequent uptake by recipient cells remain poorly understood. In this review, we aim to summarize current insights and advancements in the biogenesis and uptake mechanisms of EVs. By shedding light on the fundamental mechanisms governing EV biogenesis and delivery, this review underscores the potential of basic mechanistic research to pave the way for developing novel diagnostic strategies and therapeutic applications.
Keywords: biogenesis; delivery; ectosomes; exosomes; extracellular vesicles; network analysis; uptake.
Copyright © 2024 Yu, Sane, Kim, Yun, Kim, Jo, Wright, Khoshdoozmasouleh, Lee, Oh, Thiel, Parvin, Williams, Hannon, Lee and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

