In Vitro Antiviral Activity of a New Indol-3-carboxylic Acid Derivative Against SARS-CoV-2
- PMID: 38234608
- PMCID: PMC10790354
- DOI: 10.32607/actanaturae.26623
In Vitro Antiviral Activity of a New Indol-3-carboxylic Acid Derivative Against SARS-CoV-2
Abstract
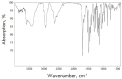
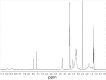
The coronavirus disease (COVID-19) pandemic has brought into sharp relief the threat posed by coronaviruses and laid the foundation for a fundamental analysis of this viral family, as well as a search for effective anti-COVID drugs. Work is underway to update existent vaccines against COVID-19, and screening for low-molecular-weight anti-COVID drug candidates for outpatient medicine continues. The opportunities and ways to accelerate the development of antiviral drugs against other pathogens are being discussed in the context of preparing for the next pandemic. In 2012-2015, Tsyshkova et al. synthesized a group of water-soluble low-molecular-weight compounds exhibiting an antiviral activity, whose chemical structure was similar to that of arbidol. Among those, there were a number of water-soluble compounds based on 5-methoxyindole-3-carboxylic acid aminoalkyl esters. Only one member of this rather extensive group of compounds, dihydrochloride of 6-bromo-5-methoxy-1-methyl-2-(1-piperidinomethyl)-3-(2-diethylaminoethoxy) carbonylindole, exhibited a reliable antiviral effect against SARS-CoV-2 in vitro. At a concentration of 52.0 μM, this compound completely inhibited the replication of the SARS-CoV-2 virus with an infectious activity of 106 TCID50/mL. The concentration curves of the analyzed compound indicate the specificity of its action. Interferon-inducing activity, as well as suppression of syncytium formation induced by the spike protein (S-glycoprotein) of SARS-CoV-2 by 89%, were also revealed. In view of its synthetic accessibility - high activity (IC50 = 1.06 μg/mL) and high selectivity index (SI = 78.6) - this compound appears to meets the requirements for the development of antiviral drugs for COVID-19 prevention and treatment.
Keywords: SARS-CoV-2; antiviral activity; cell culture; indole-3 carboxylic acid derivative.
Copyright ® 2023 National Research University Higher School of Economics.
Figures




References
-
- WHO has announced the end of the coronavirus pandemic. https://www.rbc.ru/society/05/05/2023/645503499a79477d05bf2bb4 RBC.
-
- https://covid19.who.int/ WHO Coronavirus (COVID-19) Dashboard.
-
- Media briefing on COVID-19 and global health issues. https://www.youtube.com/watch?v=B0oBevft4bs World Health Organization (WHO).
-
- Crook H., Raza S., Nowell J., Young M., Edison P., BMJ. 2021;374(1648):1–18. - PubMed
-
- Coopersmith C.M., Antonelli M., Bauer S.R., Deutschman C.S., Evans L.E., Ferrer R., Hellman J., Jog S., Kesecioglu J., Kissoon N.. Crit. Care Med. 2021;49(4):598–622. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
