This is a preprint.
Small vs. Large Library Docking for Positive Allosteric Modulators of the Calcium Sensing Receptor
- PMID: 38234749
- PMCID: PMC10793424
- DOI: 10.1101/2023.12.27.573448
Small vs. Large Library Docking for Positive Allosteric Modulators of the Calcium Sensing Receptor
Update in
-
Large library docking identifies positive allosteric modulators of the calcium-sensing receptor.Science. 2024 Sep 20;385(6715):eado1868. doi: 10.1126/science.ado1868. Epub 2024 Sep 20. Science. 2024. PMID: 39298584 Free PMC article.
Abstract
Drugs acting as positive allosteric modulators (PAMs) to enhance the activation of the calcium sensing receptor (CaSR) and to suppress parathyroid hormone (PTH) secretion can treat hyperparathyroidism but suffer from side effects including hypocalcemia and arrhythmias. Seeking new CaSR modulators, we docked libraries of 2.7 million and 1.2 billion molecules against transforming pockets in the active-state receptor dimer structure. Consistent with simulations suggesting that docking improves with library size, billion-molecule docking found new PAMs with a hit rate that was 2.7-fold higher than the million-molecule library and with hits up to 37-fold more potent. Structure-based optimization of ligands from both campaigns led to nanomolar leads, one of which was advanced to animal testing. This PAM displays 100-fold the potency of the standard of care, cinacalcet, in ex vivo organ assays, and reduces serum PTH levels in mice by up to 80% without the hypocalcemia typical of CaSR drugs. Cryo-EM structures with the new PAMs show that they induce residue rearrangements in the binding pockets and promote CaSR dimer conformations that are closer to the G-protein coupled state compared to established drugs. These findings highlight the promise of large library docking for therapeutic leads, especially when combined with experimental structure determination and mechanism.
Conflict of interest statement
Competing Interests: BKS is a founder of Epiodyne, Inc, BlueDolphin, LLC, and Deep Apple Therapeutics, Inc., serves on the SAB of Schrodinger LLC and of Vilya Therapeutics, on the SRB of Genentech, and consults for Levator Therapeutics and Hyku Therapeutics. GS is a founder and consultant of Deep Apple Therapeutics, Inc. JJI co-founded Deep Apple Therapeutics, Inc., and BlueDolphin, LLC.
Figures


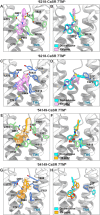

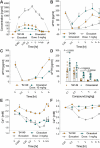
References
-
- Brown E. M. et al. , Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366, 575–580 (1993). - PubMed
-
- Hannan F. M., Thakker R. V., Calcium-sensing receptor (CaSR) mutations and disorders of calcium, electrolyte and water metabolism. Best Pract Res Clin Endocrinol Metab 27, 359–371 (2013). - PubMed
-
- Pollak M. R. et al. , Autosomal dominant hypocalcaemia caused by a Ca(2+)-sensing receptor gene mutation. Nat Genet 8, 303–307 (1994). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
