This is a preprint.
A Combination of Four Nuclear Targeted Effectors Protects Toxoplasma Against Interferon Gamma Driven Human Host Cell Death During Acute Infection
- PMID: 38234811
- PMCID: PMC10793417
- DOI: 10.1101/2023.12.24.573224
A Combination of Four Nuclear Targeted Effectors Protects Toxoplasma Against Interferon Gamma Driven Human Host Cell Death During Acute Infection
Update in
-
A combination of four Toxoplasma gondii nuclear-targeted effectors protects against interferon gamma-driven human host cell death.mBio. 2024 Oct 16;15(10):e0212424. doi: 10.1128/mbio.02124-24. Epub 2024 Sep 18. mBio. 2024. PMID: 39292011 Free PMC article.
Abstract
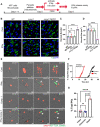
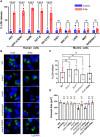
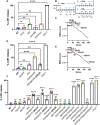
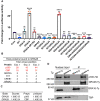
In both mice and humans, Type II interferon-gamma (IFNγ) is crucial for regulation of Toxoplasma gondii (T. gondii) infection, during acute or chronic phases. To thwart this defense, T. gondii secretes protein effectors hindering the hosťs immune response. For example, T. gondii relies on the MYR translocon complex to deploy soluble dense granule effectors (GRAs) into the host cell cytosol or nucleus. Recent genome-wide loss-of-function screens in IFNγ-primed primary human fibroblasts identified MYR translocon components as crucial for parasite resistance against IFNγ driven vacuole clearance. However, these screens did not pinpoint specific MYR-dependent GRA proteins responsible for IFNγ signaling blockade, suggesting potential functional redundancy. Our study reveals that T. gondii depends on the MYR translocon complex to prevent host cell death and parasite premature egress in human cells stimulated with IFNγ postinfection, a unique phenotype observed in various human cell lines but not in murine cells. Intriguingly, inhibiting parasite egress did not prevent host cell death, indicating this mechanism is distinct from those described previously. Genome-wide loss-of-function screens uncovered TgIST, GRA16, GRA24, and GRA28 as effectors necessary for a complete block of IFNγ response. GRA24 and GRA28 directly influenced IFNγ driven transcription, GRA24's action depended on its interaction with p38 MAPK, while GRA28 disrupted histone acetyltransferase activity of CBP/p300. Given the intricate nature of the immune response to T. gondii, it appears that the parasite has evolved equally elaborate mechanisms to subvert IFNγ signaling, extending beyond direct interference with the JAK/STAT1 pathway, to encompass other signaling pathways as well.
Conflict of interest statement
Conflicts: The authors declare no conflicts.
Figures
Similar articles
-
A combination of four Toxoplasma gondii nuclear-targeted effectors protects against interferon gamma-driven human host cell death.mBio. 2024 Oct 16;15(10):e0212424. doi: 10.1128/mbio.02124-24. Epub 2024 Sep 18. mBio. 2024. PMID: 39292011 Free PMC article.
-
Hypermigration of macrophages through the concerted action of GRA effectors on NF-κB/p38 signaling and host chromatin accessibility potentiates Toxoplasma dissemination.mBio. 2024 Oct 16;15(10):e0214024. doi: 10.1128/mbio.02140-24. Epub 2024 Aug 29. mBio. 2024. PMID: 39207098 Free PMC article.
-
Toxoplasma gondii GRA28 Is Required for Placenta-Specific Induction of the Regulatory Chemokine CCL22 in Human and Mouse.mBio. 2021 Dec 21;12(6):e0159121. doi: 10.1128/mBio.01591-21. Epub 2021 Nov 16. mBio. 2021. PMID: 34781732 Free PMC article.
-
Toxoplasma gondii manipulates host cell signaling pathways via its secreted effector molecules.Parasitol Int. 2021 Aug;83:102368. doi: 10.1016/j.parint.2021.102368. Epub 2021 Apr 24. Parasitol Int. 2021. PMID: 33905814 Review.
-
Secreted Effectors Modulating Immune Responses to Toxoplasma gondii.Life (Basel). 2021 Sep 20;11(9):988. doi: 10.3390/life11090988. Life (Basel). 2021. PMID: 34575137 Free PMC article. Review.
References
-
- Webster JP. 2010. Dubey J.P. Toxoplasmosis of Animals and Humans. Parasites & Vectors 3:112.
-
- Sibley LD, Ajioka JW. 2008. Population structure of Toxoplasma gondii: clonal expansion driven by infrequent recombination and selective sweeps. Annu Rev Microbiol 62:329–51. - PubMed
-
- Clough B, Frickel EM. 2017. The Toxoplasma Parasitophorous Vacuole: An Evolving Host-Parasite Frontier. Trends Parasitol 33:473–488. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
