IntelliCage: the development and perspectives of a mouse- and user-friendly automated behavioral test system
- PMID: 38235003
- PMCID: PMC10793385
- DOI: 10.3389/fnbeh.2023.1270538
IntelliCage: the development and perspectives of a mouse- and user-friendly automated behavioral test system
Abstract
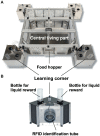
IntelliCage for mice is a rodent home-cage equipped with four corner structures harboring symmetrical double panels for operant conditioning at each of the two sides, either by reward (access to water) or by aversion (non-painful stimuli: air-puffs, LED lights). Corner visits, nose-pokes and actual licks at bottle-nipples are recorded individually using subcutaneously implanted transponders for RFID identification of up to 16 adult mice housed in the same home-cage. This allows for recording individual in-cage activity of mice and applying reward/punishment operant conditioning schemes in corners using workflows designed on a versatile graphic user interface. IntelliCage development had four roots: (i) dissatisfaction with standard approaches for analyzing mouse behavior, including standardization and reproducibility issues, (ii) response to handling and housing animal welfare issues, (iii) the increasing number of mouse models had produced a high work burden on classic manual behavioral phenotyping of single mice. and (iv), studies of transponder-chipped mice in outdoor settings revealed clear genetic behavioral differences in mouse models corresponding to those observed by classic testing in the laboratory. The latter observations were important for the development of home-cage testing in social groups, because they contradicted the traditional belief that animals must be tested under social isolation to prevent disturbance by other group members. The use of IntelliCages reduced indeed the amount of classic testing remarkably, while its flexibility was proved in a wide range of applications worldwide including transcontinental parallel testing. Essentially, two lines of testing emerged: sophisticated analysis of spontaneous behavior in the IntelliCage for screening of new genetic models, and hypothesis testing in many fields of behavioral neuroscience. Upcoming developments of the IntelliCage aim at improved stimulus presentation in the learning corners and videotracking of social interactions within the IntelliCage. Its main advantages are (i) that mice live in social context and are not stressfully handled for experiments, (ii) that studies are not restricted in time and can run in absence of humans, (iii) that it increases reproducibility of behavioral phenotyping worldwide, and (iv) that the industrial standardization of the cage permits retrospective data analysis with new statistical tools even after many years.
Keywords: animal welfare; automated behavioral analysis; comparative and evolutionary neuroscience; ethology and ecology; home-cage testing; marmoset (Callithrix jacchus); reproducibility; standardization.
Copyright © 2024 Lipp, Krackow, Turkes, Benner, Endo and Russig.
Conflict of interest statement
H-PL was representative of the consulting company Neurospex GmbH and of the software company XBehavior GmbH in Bänk (Switzerland), HR was employed by the TSE-Systems International GmbH in Berlin (Germany) and TE was representative of the Phenovance LLC in Chiba (Japan). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










Similar articles
-
Modifying reaction time tasks parameters in the automated IntelliCage identifies heightened impulsivity and impaired attention in the 3xTg-AD model of Alzheimer's disease.Front Aging Neurosci. 2024 Dec 18;16:1466415. doi: 10.3389/fnagi.2024.1466415. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39744522 Free PMC article.
-
Environmental enrichment improves hippocampus-dependent spatial learning in female C57BL/6 mice in novel IntelliCage sweet reward-based behavioral tests.Front Behav Neurosci. 2023 Sep 18;17:1256744. doi: 10.3389/fnbeh.2023.1256744. eCollection 2023. Front Behav Neurosci. 2023. PMID: 37791111 Free PMC article.
-
A sorting system with automated gates permits individual operant experiments with mice from a social home cage.J Neurosci Methods. 2011 Mar 30;196(2):276-80. doi: 10.1016/j.jneumeth.2011.01.017. Epub 2011 Jan 21. J Neurosci Methods. 2011. PMID: 21256865
-
The IntelliCage System: A Review of Its Utility as a Novel Behavioral Platform for a Rodent Model of Substance Use Disorder.Front Behav Neurosci. 2021 Jun 4;15:683780. doi: 10.3389/fnbeh.2021.683780. eCollection 2021. Front Behav Neurosci. 2021. PMID: 34149373 Free PMC article. Review.
-
Measuring Locomotor Activity and Behavioral Aspects of Rodents Living in the Home-Cage.Front Behav Neurosci. 2022 Apr 7;16:877323. doi: 10.3389/fnbeh.2022.877323. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35464142 Free PMC article. Review.
Cited by
-
Refinement of IntelliCage protocols for complex cognitive tasks through replacement of drinking restrictions by incentive-disincentive paradigms.Front Behav Neurosci. 2023 Nov 16;17:1232546. doi: 10.3389/fnbeh.2023.1232546. eCollection 2023. Front Behav Neurosci. 2023. PMID: 38033480 Free PMC article.
-
Next-Generation Cognitive-Behavioral Therapy for Depression: Integrating Digital Tools, Teletherapy, and Personalization for Enhanced Mental Health Outcomes.Medicina (Kaunas). 2025 Feb 28;61(3):431. doi: 10.3390/medicina61030431. Medicina (Kaunas). 2025. PMID: 40142242 Free PMC article.
-
Modifying reaction time tasks parameters in the automated IntelliCage identifies heightened impulsivity and impaired attention in the 3xTg-AD model of Alzheimer's disease.Front Aging Neurosci. 2024 Dec 18;16:1466415. doi: 10.3389/fnagi.2024.1466415. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39744522 Free PMC article.
-
Environmental enrichment: a systematic review on the effect of a changing spatial complexity on hippocampal neurogenesis and plasticity in rodents, with considerations for translation to urban and built environments for humans.Front Neurosci. 2024 Jun 11;18:1368411. doi: 10.3389/fnins.2024.1368411. eCollection 2024. Front Neurosci. 2024. PMID: 38919908 Free PMC article.
-
Robotic animals as new tools in rodent neuroscience research: proposed applications of zooinspired robots for mouse behavioral testing.Front Behav Neurosci. 2025 Feb 24;19:1545352. doi: 10.3389/fnbeh.2025.1545352. eCollection 2025. Front Behav Neurosci. 2025. PMID: 40066371 Free PMC article.
References
-
- Alboni S., Poggini S., Garofalo S., Milior G., El Hajj H., Lecours C., et al. . (2016). Fluoxetine treatment affects the inflammatory response and microglial function according to the quality of the living environment. Brain Behav. Immun. 58, 261–271. doi: 10.1016/j.bbi.2016.07.155, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

