Structural DNA nanotechnology at the nexus of next-generation bio-applications: challenges and perspectives
- PMID: 38235105
- PMCID: PMC10790967
- DOI: 10.1039/d3na00692a
Structural DNA nanotechnology at the nexus of next-generation bio-applications: challenges and perspectives
Abstract
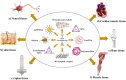
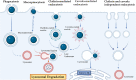
DNA nanotechnology has significantly progressed in the last four decades, creating nucleic acid structures widely used in various biological applications. The structural flexibility, programmability, and multiform customization of DNA-based nanostructures make them ideal for creating structures of all sizes and shapes and multivalent drug delivery systems. Since then, DNA nanotechnology has advanced significantly, and numerous DNA nanostructures have been used in biology and other scientific disciplines. Despite the progress made in DNA nanotechnology, challenges still need to be addressed before DNA nanostructures can be widely used in biological interfaces. We can open the door for upcoming uses of DNA nanoparticles by tackling these issues and looking into new avenues. The historical development of various DNA nanomaterials has been thoroughly examined in this review, along with the underlying theoretical underpinnings, a summary of their applications in various fields, and an examination of the current roadblocks and potential future directions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
We declare no conflict of interest.
Figures


Similar articles
-
Building DNA nanostructures for molecular computation, templated assembly, and biological applications.Acc Chem Res. 2014 Jun 17;47(6):1778-88. doi: 10.1021/ar500023b. Epub 2014 Apr 10. Acc Chem Res. 2014. PMID: 24720350
-
DNA Nanostructures: Current Challenges and Opportunities for Cellular Delivery.ACS Nano. 2021 Mar 23;15(3):3631-3645. doi: 10.1021/acsnano.0c06136. Epub 2021 Feb 26. ACS Nano. 2021. PMID: 33635620 Review.
-
Gene Therapy Based on Nucleic Acid Nanostructure.Adv Healthc Mater. 2020 Oct;9(19):e2001046. doi: 10.1002/adhm.202001046. Epub 2020 Aug 31. Adv Healthc Mater. 2020. PMID: 32864890 Review.
-
DNA nanomaterials for preclinical imaging and drug delivery.J Control Release. 2016 Oct 10;239:27-38. doi: 10.1016/j.jconrel.2016.08.013. Epub 2016 Aug 13. J Control Release. 2016. PMID: 27527555 Free PMC article. Review.
-
DNA Nanotechnology-Based Biosensors and Therapeutics.Adv Healthc Mater. 2021 Aug;10(15):e2002205. doi: 10.1002/adhm.202002205. Epub 2021 Jun 3. Adv Healthc Mater. 2021. PMID: 34085411 Review.
Cited by
-
Lateral nanoarchitectonics from nano to life: ongoing challenges in interfacial chemical science.Chem Sci. 2024 Oct 28;15(45):18715-18750. doi: 10.1039/d4sc05575f. eCollection 2024 Nov 20. Chem Sci. 2024. PMID: 39568623 Free PMC article. Review.
-
The Application of Ultrasound Pre-Treatment in Low-Temperature Synthesis of Zinc Oxide Nanorods.Materials (Basel). 2024 Oct 11;17(20):4980. doi: 10.3390/ma17204980. Materials (Basel). 2024. PMID: 39459685 Free PMC article.
-
Nanomolecular silencing of TSC22D4 mRNA via a DNAsome-siRNA for enhancing insulin sensitization in hepatocytes.Iran J Basic Med Sci. 2025;28(3):385-392. doi: 10.22038/ijbms.2024.81998.17744. Iran J Basic Med Sci. 2025. PMID: 39906612 Free PMC article.
-
In Vivo Interactions of Nucleic Acid Nanostructures With Cells.Adv Mater. 2025 Jan;37(2):e2314232. doi: 10.1002/adma.202314232. Epub 2024 Sep 12. Adv Mater. 2025. PMID: 39263835 Free PMC article. Review.
-
Supramolecular chemistry for optical detection and delivery applications in living plants.Chem Soc Rev. 2025 Aug 26;54(17):7769-7869. doi: 10.1039/d4cs00500g. Chem Soc Rev. 2025. PMID: 40673397 Free PMC article. Review.
References
-
- Zhou Y. Dong J. Wang Q. NPG Asia Mater. 2023;15:25. doi: 10.1038/s41427-023-00470-3. - DOI
Publication types
LinkOut - more resources
Full Text Sources

