Platelet derived exosomes disrupt endothelial cell monolayer integrity and enhance vascular inflammation in dengue patients
- PMID: 38235130
- PMCID: PMC10791899
- DOI: 10.3389/fimmu.2023.1285162
Platelet derived exosomes disrupt endothelial cell monolayer integrity and enhance vascular inflammation in dengue patients
Abstract
Background: Thrombocytopenia is the most notable phenomenon in dengue. Activation status of platelets and interaction of platelets with endothelium contribute towards dengue disease pathogenesis. Platelets are the major cell types known to release extracellular vesicles, especially exosomes in circulation. However, the role of platelet derived exosomes (PLT-EXOs) in endothelial dysfunction during dengue infection remains unknown.
Methods: In this study, we recruited 28 healthy subjects and 69 dengue patients categorized as WS- (n=31), WS+ (n=29) and SD (n=9). Platelets were isolated from platelet rich plasma of dengue patients and their activation was assessed by flow cytometry. PLT-EXOs were isolated by ultracentrifugation method. Western blot analyses were performed to characterize the exosomes. Exosome uptake experiment was carried out to see the internalization of exosomes inside endothelial cells (HUVECs). To observe the effect of exosomes on endothelial cells, exosomes were added on HUVECs and expression of adherens and tight junctional proteins were examined by immunofluorescence assay and western blot. Expression levels of vascular injury markers were measured in the culture supernatants of Exosome-HUVEC coculture and sera of dengue patients by MSD-multiplex assay.
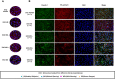
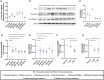
Results: As compared to healthy subjects, CD41/CD61 expression was significantly reduced (p<0.0001) and CD62p expression was significantly increased (p<0.0001) on platelets in dengue patients. PLT-EXOs isolated from the dengue patients showed higher expression of CD63 and CD9 proteins than the healthy subjects. With in-vitro immunofluorescence assays, we illustrated the internalization of PLT-EXOs by the HUVECs and observed disruption of endothelial cell monolayer integrity in the presence of PLT-EXOs from WS+ and SD patients. Furthermore, the significant reduction in the expressions of ZO-2, VE-Cadherin and CD31 in endothelial cells following exposure to PLT-EXOs from the dengue patients provide direct evidence of PLT-EXOs mediated vascular permeability. PLT-EXOs stimulated the release of inflammatory markers CRP, SAA, sVCAM-1 and sICAM-1 in the supernatants of HUVEC cells. Importantly, significantly higher levels of CRP, sVCAM-1 and sICAM-1 in the sera of severe than mild dengue patients (p<0.0001) suggest their role in disease severity.
Conclusions: In summary, our data suggest that PLT-EXOs promote vascular leakage via release of proinflammatory mediators and compromise vascular barrier integrity in dengue patients.
Keywords: dengue virus; exosomes; platelet-derived exosomes; platelets; sVCAM-1; vascular inflammation.
Copyright © 2024 Vedpathak, Sharma, Palkar, Bhatt, Patil, Kakrani, Mishra, Bhosle, Arankalle and Shrivastava.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






References
-
- World Health Organization . Dengue: Guidelines for diagnosis, treatment, prevention and control. Geneva, Switzerland: World Health Organization; (2009). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

