Riboproteome remodeling during quiescence exit in Saccharomyces cerevisiae
- PMID: 38235324
- PMCID: PMC10792236
- DOI: 10.1016/j.isci.2023.108727
Riboproteome remodeling during quiescence exit in Saccharomyces cerevisiae
Abstract
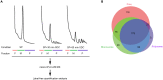
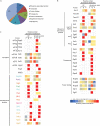
The quiescent state is the prevalent mode of cellular life in most cells. Saccharomyces cerevisiae is a useful model for studying the molecular basis of the cell cycle, quiescence, and aging. Previous studies indicate that heterogeneous ribosomes show a specialized translation function to adjust the cellular proteome upon a specific stimulus. Using nano LC-MS/MS, we identified 69 of the 79 ribosomal proteins (RPs) that constitute the eukaryotic 80S ribosome during quiescence. Our study shows that the riboproteome is composed of 444 accessory proteins comprising cellular functions such as translation, protein folding, amino acid and glucose metabolism, cellular responses to oxidative stress, and protein degradation. Furthermore, the stoichiometry of both RPs and accessory proteins on ribosome particles is different depending on growth conditions and among monosome and polysome fractions. Deficiency of different RPs resulted in defects of translational capacity, suggesting that ribosome composition can result in changes in translational activity during quiescence.
Keywords: Cell biology.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

