Biallelic SOX8 Variants Associated With Novel Syndrome With Myopathy, Skeletal Deformities, Intellectual Disability, and Ovarian Dysfunction
- PMID: 38235364
- PMCID: PMC10508790
- DOI: 10.1212/NXG.0000000000200088
Biallelic SOX8 Variants Associated With Novel Syndrome With Myopathy, Skeletal Deformities, Intellectual Disability, and Ovarian Dysfunction
Abstract
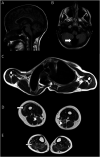
Background and objectives: The human genome contains ∼20,000 genes, each of which has its own set of complex regulatory systems to govern precise expression in each developmental stage and cell type. Here, we report a female patient with congenital weakness, respiratory failure, skeletal dysplasia, contractures, short stature, intellectual delay, respiratory failure, and amenorrhea who presented to Medical Genetics service with no known cause for her condition.
Methods: Whole-exome and whole-genome sequencing were conducted, as well as investigational functional studies to assess the effect of SOX8 variant.
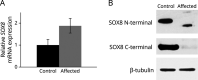
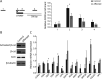
Results: The patient was found to have biallelic SOX8 variants (NM_014587.3:c.422+5G>C; c.583dup p.(His195ProfsTer11)). SOX8 is a transcriptional regulator, which is predicted to be imprinted (expressed from only one parental allele), but this has not yet been confirmed. We provide evidence that while SOX8 was maternally expressed in adult-derived fibroblasts and lymphoblasts, it was biallelically expressed in other cell types and therefore suggest that biallelic variants are associated with this recessive condition. Functionally, we showed that the paternal variant had the capacity to affect mRNA splicing while the maternal variant resulted in low levels of a truncated protein, which showed decreased binding at and altered expression of SOX8 targets.
Discussion: Our findings associate SOX8 variants with this novel condition, highlight how complex genome regulation can complicate novel disease-gene identification, and provide insight into the molecular pathogenesis of this disease.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
The authors report no relevant disclosures. Go to Neurology.org/NG for full disclosures
Figures


Similar articles
-
Genome-wide identification of Sox8-, and Sox9-dependent genes during early post-natal testis development in the mouse.Andrology. 2013 Mar;1(2):281-92. doi: 10.1111/j.2047-2927.2012.00049.x. Epub 2013 Jan 13. Andrology. 2013. PMID: 23315995
-
Variation analysis of SOX8 gene in Chinese men with non-obstructive azoospermia or oligozoospermia.Andrologia. 2020 May;52(4):e13531. doi: 10.1111/and.13531. Epub 2020 Feb 12. Andrologia. 2020. PMID: 32048324
-
Characterization of a novel deep-intronic variant in DYNC2H1 identified by whole-exome sequencing in a patient with a lethal form of a short-rib thoracic dysplasia type III.Cold Spring Harb Mol Case Stud. 2022 Dec 28;8(7):a006254. doi: 10.1101/mcs.a006254. Print 2022 Dec. Cold Spring Harb Mol Case Stud. 2022. PMID: 36442996 Free PMC article.
-
Craniofacial dysmorphism, skeletal anomalies, and impaired intellectual development syndrome-1 in two new patients with the same homozygous TMCO1 variant and review of the literature.Eur J Med Genet. 2023 Mar;66(3):104715. doi: 10.1016/j.ejmg.2023.104715. Epub 2023 Jan 25. Eur J Med Genet. 2023. PMID: 36708876 Review.
-
Origin and possible roles of the Sox8 transcription factor gene during sexual development.Cytogenet Genome Res. 2003;101(3-4):212-8. doi: 10.1159/000074339. Cytogenet Genome Res. 2003. PMID: 14689607 Review.
Cited by
-
Sox8: a multifaceted transcription factor in development and disease.Biol Open. 2025 Feb 15;14(2):bio061840. doi: 10.1242/bio.061840. Epub 2025 Feb 12. Biol Open. 2025. PMID: 39936824 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
