Nernst-Planck-Gaussian modelling of electrodiffusional recovery from ephaptic excitation between mammalian cardiomyocytes
- PMID: 38235384
- PMCID: PMC10791825
- DOI: 10.3389/fphys.2023.1280151
Nernst-Planck-Gaussian modelling of electrodiffusional recovery from ephaptic excitation between mammalian cardiomyocytes
Abstract
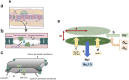
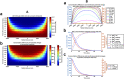
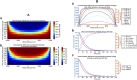
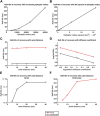
Introduction: In addition to gap junction conduction, recent reports implicate possible ephaptic coupling contributions to action potential (AP) propagation between successive adjacent cardiomyocytes. Here, AP generation in an active cell, withdraws Na+ from, creating a negative potential within, ephaptic spaces between the participating membranes, activating the initially quiescent neighbouring cardiomyocyte. However, sustainable ephaptic transmission requires subsequent complete recovery of the ephaptic charge difference. We explore physical contributions of passive electrodiffusive ion exchange with the remaining extracellular space to this recovery for the first time. Materials and Methods: Computational, finite element, analysis examined limiting, temporal and spatial, ephaptic [Na+], [Cl-], and the consequent Gaussian charge differences and membrane potential recovery patterns following a ΔV∼130 mV AP upstroke at physiological (37°C) temperatures. This incorporated Nernst-Planck formalisms into equations for the time-dependent spatial concentration gradient profiles. Results: Mammalian atrial, ventricular and purkinje cardiomyocyte ephaptic junctions were modelled by closely apposed circularly symmetric membranes, specific capacitance 1 μF cm-2, experimentally reported radii a = 8,000, 12,000 and 40,000 nm respectively and ephaptic axial distance w = 20 nm. This enclosed an ephaptic space containing principal ions initially at normal extracellular [Na+] = 153.1 mM and [Cl-] = 145.8 mM, respective diffusion coefficients D Na = 1.3 109 and D Cl = 2 109 nm2s-1. Stable, concordant computational solutions were confirmed exploring ≤1,600 nm mesh sizes and Δt≤0.08 ms stepsize intervals. The corresponding membrane voltage profile changes across the initially quiescent membrane were obtainable from computed, graphically represented a and w-dependent ionic concentration differences adapting Gauss's flux theorem. Further simulations explored biological variations in ephaptic dimensions, membrane anatomy, and diffusion restrictions within the ephaptic space. Atrial, ventricular and Purkinje cardiomyocytes gave 40, 180 and 2000 ms 99.9% recovery times, with 720 or 360 ms high limits from doubling ventricular radius or halving diffusion coefficient. Varying a, and D Na and D Cl markedly affected recovery time-courses with logarithmic and double-logarithmic relationships, Varying w exerted minimal effects. Conclusion: We thereby characterise the properties of, and through comparing atrial, ventricular and purkinje recovery times with interspecies in vivo background cardiac cycle duration data, (blue whale ∼2000, human∼90, Etruscan shrew, ∼40 ms) can determine physical limits to, electrodiffusive contributions to ephaptic recovery.
Keywords: action potential propagation; cardiomyocytes; electrodiffusion; ephaptic conduction; sodium channels.
Copyright © 2024 Morris, Bardsley, Salvage, Jackson, Matthews and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Nernst-Planck-Gaussian finite element modelling of Ca2+ electrodiffusion in amphibian striated muscle transverse tubule-sarcoplasmic reticular triadic junctional domains.Front Physiol. 2024 Dec 5;15:1468333. doi: 10.3389/fphys.2024.1468333. eCollection 2024. Front Physiol. 2024. PMID: 39703671 Free PMC article.
-
Localization of Na+ channel clusters in narrowed perinexi of gap junctions enhances cardiac impulse transmission via ephaptic coupling: a model study.J Physiol. 2021 Nov;599(21):4779-4811. doi: 10.1113/JP282105. Epub 2021 Oct 4. J Physiol. 2021. PMID: 34533834 Free PMC article.
-
Distribution of cardiac sodium channels in clusters potentiates ephaptic interactions in the intercalated disc.J Physiol. 2018 Feb 15;596(4):563-589. doi: 10.1113/JP275351. Epub 2018 Jan 9. J Physiol. 2018. PMID: 29210458 Free PMC article.
-
Gap junctional and ephaptic coupling in cardiac electrical propagation: homocellular and heterocellular perspectives.J Physiol. 2025 May 31. doi: 10.1113/JP287358. Online ahead of print. J Physiol. 2025. PMID: 40448893 Review.
-
The role of the gap junction perinexus in cardiac conduction: Potential as a novel anti-arrhythmic drug target.Prog Biophys Mol Biol. 2019 Jul;144:41-50. doi: 10.1016/j.pbiomolbio.2018.08.003. Epub 2018 Sep 19. Prog Biophys Mol Biol. 2019. PMID: 30241906 Free PMC article. Review.
Cited by
-
Cardiac arrhythmogenesis: roles of ion channels and their functional modification.Front Physiol. 2024 Mar 4;15:1342761. doi: 10.3389/fphys.2024.1342761. eCollection 2024. Front Physiol. 2024. PMID: 38505707 Free PMC article. Review.
-
Nernst-Planck-Gaussian finite element modelling of Ca2+ electrodiffusion in amphibian striated muscle transverse tubule-sarcoplasmic reticular triadic junctional domains.Front Physiol. 2024 Dec 5;15:1468333. doi: 10.3389/fphys.2024.1468333. eCollection 2024. Front Physiol. 2024. PMID: 39703671 Free PMC article.
-
New focus on cardiac voltage-gated sodium channel β1 and β1B: Novel targets for treating and understanding arrhythmias?Heart Rhythm. 2025 Jan;22(1):181-191. doi: 10.1016/j.hrthm.2024.06.029. Epub 2024 Jun 21. Heart Rhythm. 2025. PMID: 38908461 Free PMC article. Review.
References
-
- Benedict F. G., Lee R. C. (1936). The heart rate of the elephant. Proc. Am. Philos. Soc. 76, 335–341. Available at: http://www.jstor.org/stable/984548.
-
- Bennett M. (1977). “Electrical transmission: a functional analysis and comparison to chemical transmission,” in Handbook of Physiology. Editor Kandel E. (Bethesda, MD: American Physiological Society; ), 357–416.
LinkOut - more resources
Full Text Sources
Miscellaneous

