Prolonged hypothermic machine perfusion enables daytime liver transplantation - an IDEAL stage 2 prospective clinical trial
- PMID: 38235423
- PMCID: PMC10789636
- DOI: 10.1016/j.eclinm.2023.102411
Prolonged hypothermic machine perfusion enables daytime liver transplantation - an IDEAL stage 2 prospective clinical trial
Abstract
Background: Liver transplantation is traditionally performed around the clock to minimize organ ischemic time. However, the prospect of prolonging preservation times holds the potential to streamline logistics and transform liver transplantation into a semi-elective procedure, reducing the need for nighttime surgeries. Dual hypothermic oxygenated machine perfusion (DHOPE) of donor livers for 1-2 h mitigates ischemia-reperfusion injury and improves transplant outcomes. Preclinical studies have shown that DHOPE can safely extend the preservation of donor livers for up to 24 h.
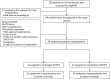
Methods: We conducted an IDEAL stage 2 prospective clinical trial comparing prolonged (≥4 h) DHOPE to conventional (1-2 h) DHOPE for brain-dead donor livers, enabling transplantation the following morning. Liver allocation to each group was based on donor hepatectomy end times. The primary safety endpoint was a composite of all serious adverse events (SAE) within 30 days after transplantation. The primary feasibility endpoint was defined as the number of patients assigned and successfully receiving a prolonged DHOPE-perfused liver graft. Trial registration at: WHO International Clinical Trial Registry Platform, number NL8740.
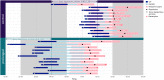
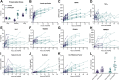
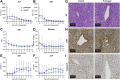
Findings: Between November 1, 2020 and July 16, 2022, 24 patients were enrolled. The median preservation time was 14.5 h (interquartile range [IQR], 13.9-15.5) for the prolonged group (n = 12) and 7.9 h (IQR, 7.6-8.6) for the control group (n = 12; p = 0.01). In each group, three patients (25%; 95% CI 3.9-46%, p = 1) experienced a SAE. Markers of ischemia-reperfusion injury and oxidative stress in both perfusate and recipients were consistently low and showed no notable discrepancies between the two groups. All patients assigned to either the prolonged group or control group successfully received a liver graft perfused with either prolonged DHOPE or control DHOPE, respectively.
Interpretation: This first-in-human clinical trial demonstrates the safety and feasibility of DHOPE in prolonging the preservation time of donor livers to enable daytime transplantation. The ability to extend the preservation window to up to 20 h using hypothermic oxygenated machine preservation at a 10 °C temperature has the potential to reshape the landscape of liver transplantation.
Funding: University Medical Center Groningen, the Netherlands.
Keywords: Clinical trial; Hypothermic machine perfusion; IDEAL stage 2; Liver transplantation; Machine perfusion; Machine preservation.
© 2023 The Author(s).
Conflict of interest statement
Vincent E. de Meijer reports a VENI research grant by the Dutch Research Council (NWO; grant #09150161810030), a Research grant from the Dutch Ministry of Economic Affairs (Health ∼ Holland Public Private Partnership grant #PPP-2019-024), and a Research grant from the Dutch Society for Gastroenterology (NVGE #01-2021), all outside the submitted work. Other authors declare that they have no competing interests.
Figures

References
-
- Cardini B., Oberhuber R., Fodor M., et al. Clinical implementation of prolonged liver preservation and monitoring through normothermic machine perfusion in liver transplantation. Transplantation. 2020;104(9):1917–1928. - PubMed
-
- de Meijer V.E., Fujiyoshi M., Porte R.J. Ex situ machine perfusion strategies in liver transplantation. J Hepatol. 2019;70(1):203–205. - PubMed
-
- van Rijn R., Schurink I.J., de Vries Y., et al. Hypothermic machine perfusion in liver transplantation — a randomized trial. N Engl J Med. 2021;384(15):1391–1401. - PubMed
-
- Czigany Z., Pratschke J., Froněk J., et al. Hypothermic oxygenated machine perfusion (HOPE) reduces early allograft injury and improves post-transplant outcomes in extended criteria donation (ECD) liver transplantation from donation after brain death (DBD): results from a multicenter randomized controlled trial (HOPE ECD-DBD) Ann Surg. 2021;274:705. - PubMed
LinkOut - more resources
Full Text Sources

