Characterization of antibody-dependent cellular phagocytosis in patients infected with hepatitis C virus with different clinical outcomes
- PMID: 38235622
- PMCID: PMC10953302
- DOI: 10.1002/jmv.29381
Characterization of antibody-dependent cellular phagocytosis in patients infected with hepatitis C virus with different clinical outcomes
Abstract
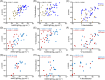
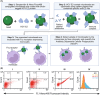
Early neutralizing antibodies against hepatitis C virus (HCV) and CD8 + T cell effector responses can lead to viral clearance. However, these functions alone are not sufficient to protect patients against HCV infection, thus undefined additional antiviral immune mechanisms are required. In recent years, Fc-receptor-dependent antibody effector functions, particularly, antibody-dependent cellular phagocytosis (ADCP) were shown to offer immune protection against several RNA viruses. However, its development and clinical role in patients with HCV infection remain unknown. In this study, we found that patients with chronic GT1a or GT3a HCV infection had significantly higher concentrations of anti-envelope 2 (E2) antibodies, predominantly IgG1 subclass, than patients that cleared the viruses while the latter had antibodies with higher affinities. 97% of the patients with HCV had measurable ADCP of whom patients with chronic disease showed significantly higher ADCP than those who naturally cleared the virus. Epitope mapping studies showed that patients with antibodies that target antigenic domains on the HCV E2 protein that are known to associate with neutralization function are also strongly associated with ADCP, suggesting antibodies with overlapping/dual functions. Correlation studies showed that ADCP significantly correlated with plasma anti-E2 antibody levels and neutralization function regardless of clinical outcome and genotype of infecting virus, while a significant correlation between ADCP and affinity was only evident in patients that cleared the virus. These results suggest ADCP was mostly driven by antibody titer in patients with chronic disease while maintained in clearers due to the quality (affinity) of their anti-E2 antibodies despite having lower antibody titers.
Keywords: affinity; epitope mapping; hepatitis C; neutralization; phagocytosis.
© 2024 The Authors. Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Optimisation and validation of a new method for antibody dependent cellular phagocytosis in hepatitis C virus infection.J Immunol Methods. 2021 Aug;495:113087. doi: 10.1016/j.jim.2021.113087. Epub 2021 Jun 18. J Immunol Methods. 2021. PMID: 34147479
-
Broadly neutralizing antibodies from an individual that naturally cleared multiple hepatitis C virus infections uncover molecular determinants for E2 targeting and vaccine design.PLoS Pathog. 2019 May 17;15(5):e1007772. doi: 10.1371/journal.ppat.1007772. eCollection 2019 May. PLoS Pathog. 2019. PMID: 31100098 Free PMC article.
-
Role of the E2 Hypervariable Region (HVR1) in the Immunogenicity of a Recombinant Hepatitis C Virus Vaccine.J Virol. 2018 May 14;92(11):e02141-17. doi: 10.1128/JVI.02141-17. Print 2018 Jun 1. J Virol. 2018. PMID: 29540595 Free PMC article.
-
Antibody-Dependent Cellular Phagocytosis in Antiviral Immune Responses.Front Immunol. 2019 Feb 28;10:332. doi: 10.3389/fimmu.2019.00332. eCollection 2019. Front Immunol. 2019. PMID: 30873178 Free PMC article. Review.
-
Neutralizing antibody response to hepatitis C virus.Viruses. 2011 Nov;3(11):2127-45. doi: 10.3390/v3112127. Epub 2011 Nov 2. Viruses. 2011. PMID: 22163337 Free PMC article. Review.
References
-
- Younossi ZM, Wong G, Anstee QM, Henry L. The global burden of liver disease. Clin Gastroenterol Hepatol. 2023;21:1978‐1991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

