Tunable control of B. infantis abundance and gut metabolites by co-administration of human milk oligosaccharides
- PMID: 38235736
- PMCID: PMC10798361
- DOI: 10.1080/19490976.2024.2304160
Tunable control of B. infantis abundance and gut metabolites by co-administration of human milk oligosaccharides
Abstract
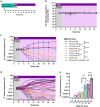
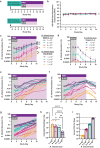
Precision engineering of the gut microbiome holds promise as an effective therapeutic approach for diseases associated with a disruption in this microbial community. Engrafting a live biotherapeutic product (LBP) in a predictable, controllable manner is key to the consistent success of this approach and has remained a challenge for most LBPs under development. We recently demonstrated high-level engraftment of Bifidobacterium longum subsp. infantis (B. infantis) in adults when co-dosed with a specific prebiotic, human milk oligosaccharides (HMO). Here, we present a cellular kinetic-pharmacodynamic approach, analogous to pharmacokinetic-pharmacodynamic-based analyses of small molecule- and biologic-based drugs, to establish how HMO controls expansion, abundance, and metabolic output of B. infantis in a human microbiota-based model in gnotobiotic mice. Our data demonstrate that the HMO dose controls steady-state abundance of B. infantis in the microbiome, and that B. infantis together with HMO impacts gut metabolite levels in a targeted, HMO-dependent manner. We also found that HMO creates a privileged niche for B. infantis expansion across a 5-log range of bacterial inocula. These results demonstrate remarkable control of both B. infantis levels and the microbiome community metabolic outputs using this synbiotic approach, and pave the way for precision engineering of desirable microbes and metabolites to treat a range of diseases.
Keywords: B. infantis; Bifidobacterium; Gut microbiome; Human milk oligosaccharides (HMO); Live biotherapeutic product (LBP); gut microbiota; microbiome modulation; synbiotic.
Conflict of interest statement
Prolacta Bioscience employees are also shareholders in the company. U.S. Patent No. 8,927,027, U.S. Patent Application No. 20200054035, International Application Pub. Nos. WO 2021/061991 and WO 2022/036225, and their corresponding family member patents and applications relate to aspects of this work.
Figures

References
-
- U.S. Food and Drug Administration, Center for Biologics Evaluation and Research . Early clinical trials with live biotherapeutic products: chemistry, manufacturing, and Control Information; Guidance for Industry. Silver Spring, MD; 2016. https://www.regulations.gov/docket/FDA-2010-D-0500.
-
- Aggarwala V, Mogno I, Li Z, Yang C, Britton GJ, Chen-Liaw A, Mitcham J, Bongers G, Gevers D, Clemente JC. et al. Precise quantification of bacterial strains after fecal microbiota transplantation delineates long-term engraftment and explains outcomes. Nat Microbiol. 2021;6(10):1309–1318. doi: 10.1038/s41564-021-00966-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
