The role of serum/glucocorticoid-regulated kinase 1 in brain function following cerebral ischemia
- PMID: 38235747
- PMCID: PMC11179613
- DOI: 10.1177/0271678X231224508
The role of serum/glucocorticoid-regulated kinase 1 in brain function following cerebral ischemia
Abstract
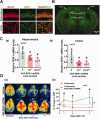
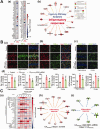
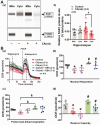
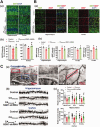
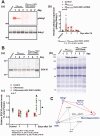
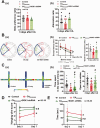
Cardiopulmonary arrest (CA) is a major cause of death/disability in the U.S. with poor prognosis and survival rates. Current therapeutic challenges are physiologically complex because they involve hypoperfusion (decreased cerebral blood flow), neuroinflammation, and mitochondrial dysfunction. We previously discovered novel serum/glucocorticoid-regulated kinase 1 (SGK1) is highly expressed in brain of neurons that are susceptible to ischemia (hippocampus and cortex). We inhibited SGK1 and utilized pharmacological (specific inhibitor, GSK650394) and neuron-specific genetic approaches (shRNA) in rodent models of CA to determine if SGK1 is responsible for hypoperfusion, neuroinflammation, mitochondrial dysfunctional, and neurological deficits after CA. Inhibition of SGK1 alleviated cortical hypoperfusion and neuroinflammation (via Iba1, GFAP, and cytokine array). Treatment with GSK650394 enhanced mitochondrial function (via Seahorse respirometry) in the hippocampus 3 and 7 days after CA. Neuronal injury (via MAP2, dMBP, and Golgi staining) in the hippocampus and cortex was observed 7 days after CA but ameliorated with SGK1-shRNA. Moreover, SGK1 mediated neuronal injury by regulating the Ndrg1-SOX10 axis. Finally, animals subjected to CA exhibited learning/memory, motor, and anxiety deficits after CA, whereas SGK1 inhibition via SGK1-shRNA improved neurocognitive function. The present study suggests the fundamental roles of SGK1 in brain circulation and neuronal survival/death in cerebral ischemia-related diseases.
Keywords: Serum/glucocorticoid-regulated kinase; cerebral ischemia; mitochondrial dysfunction; neuroinflammation; neurological deficits.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Virani SS, Alonso A, Aparicio HJ, et al.. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation 2021; 143: e254–e743. - PubMed
-
- Neumar RW, Nolan JP, Adrie C, et al.. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the international liaison committee on resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008; 118: 2452–2483. - PubMed
-
- Harukuni I, Bhardwaj A. Mechanisms of brain injury after global cerebral ischemia. Neurol Clin 2006; 24: 1–21. - PubMed
-
- Lang F, Bohmer C, Palmada M, et al.. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev 2006; 86: 1151–1178. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

