Transjugular intrahepatic portosystemic shunts for adults with hepatorenal syndrome
- PMID: 38235907
- PMCID: PMC10795102
- DOI: 10.1002/14651858.CD011039.pub2
Transjugular intrahepatic portosystemic shunts for adults with hepatorenal syndrome
Abstract
Background: Hepatorenal syndrome is a condition that occurs in people with chronic liver disease (such as alcoholic hepatitis, advanced cirrhosis, or fulminant liver failure) and portal hypertension. The prognosis is dismal, often with a survival of weeks to months. Hepatorenal syndrome is characterised by the development of intense splanchnic vasodilation favouring ascites and hypotension leading to renal vasoconstriction and acute renal failure. Therefore, treatment attempts focus on improving arterial pressure through the use of vasopressors, paracentesis, and increasing renal perfusion pressure. Several authors have reported that the placement of transjugular intrahepatic portosystemic shunts (TIPS) may be a therapeutic option because it decreases portal pressure and improves arterial and renal pressures. However, the evidence is not clearly documented and TIPS may cause adverse events. Accordingly, it is necessary to evaluate the evidence of the benefits and harms of TIPS to assess its value in people with hepatorenal syndrome.
Objectives: To evaluate the benefits and harms of transjugular intrahepatic portosystemic shunts (TIPS) in adults with hepatorenal syndrome compared with sham, no intervention, conventional treatment, or other treatments.
Search methods: We used standard, extensive Cochrane search methods. The latest search date was 2 June 2023.
Selection criteria: We included only randomised clinical trials with a parallel-group design, which compared the TIPS placement with sham, no intervention, conventional therapy, or other therapies, in adults aged 18 years or older, regardless of sex or ethnicity, diagnosed with chronic liver disease and hepatorenal syndrome. We excluded trials of adults with kidney failure due to causes not related to hepatorenal syndrome, and we also excluded data from quasi-randomised, cross-over, and observational study designs as we did not design a separate search for such studies.
Data collection and analysis: We used standard Cochrane methods. Our primary outcomes were 1. all-cause mortality, 2. morbidity due to any cause, and 3. serious adverse events. Our secondary outcomes were 1. health-related quality of life, 2. non-serious adverse events, 3. participants who did not receive a liver transplant, 4. participants without improvement in kidney function, and 5. length of hospitalisation. We performed fixed-effect and random-effects meta-analyses using risk ratio (RR) or Peto odds ratio (Peto OR), with 95% confidence intervals (CI) for the dichotomous outcomes and mean difference (MD) or standardised mean difference (SMD) for the continuous outcomes. We used GRADE to assess certainty of evidence.
Main results: We included two randomised clinical trials comparing TIPS placement (64 participants) versus conventional treatment (paracentesis plus albumin 8 g/L of removed ascites) (66 participants). The co-interventions used in the trials were dietary treatment (sodium less than 60 mmoL/day), spironolactone (300 mg/day to 400 mg/day), and furosemide (120 mg/day). Follow-up was up to 24 months. Both were multicentre trials from Spain and the USA, and Germany, conducted between 1993 and 2002. Most participants were men (aged 18 to 75 years). We are uncertain about the effect of TIPS placement compared with conventional treatment, during the first 24 months of follow-up, on all-cause mortality (RR 0.88, 95% CI 0.55 to 1.38; 2 trials, 130 participants; I2 = 58%; very low-certainty evidence) and on the development of any serious adverse event (RR 1.60, 95% CI 0.10 to 24.59; 2 trials, 130 participants; I2 = 78%; very low-certainty evidence). The use of TIPS may or may not result in a decrease in overall morbidity such as bacterial peritonitis, encephalopathy, or refractory ascites, during the first 24 months of follow-up, compared with the conventional treatment (RR 0.95, 95% CI 0.77 to 1.18; 2 trials, 130 participants; I2 = 0%; low-certainty evidence). We are uncertain about the effect of TIPS placement versus conventional treatment on the number of people who did not receive a liver transplant (RR 1.03, 95% CI 0.93 to 1.14; 2 trials, 130 participants; I2 = 0%; very low-certainty evidence) or on the length of hospitalisation (MD -20.0 days, 95% CI -39.92 to -0.08; 1 trial, 60 participants; very low-certainty evidence). Kidney function may improve in participants with TIPS placement (RR 0.53, 95% CI 0.27 to 1.02; 1 trial, 70 participants; low-certainty evidence). No trials reported health-related quality of life, non-serious adverse events, or number of participants with improvement in liver function associated with the TIPS placement. Funding No trials reported sources of commercial funding or conflicts of interest between researchers. Ongoing studies We found one ongoing trial comparing TIPS with conventional therapy (terlipressin plus albumin) and listed one study as awaiting classification as no full-text article could be found.
Authors' conclusions: TIPS placement was compared with conventional treatment, with a follow-up of 24 months, in adults with hepatorenal syndrome type 2. Based on two trials with insufficient sample size and trial limitations, we assessed the overall certainty of evidence as low or very low. We are unsure if TIPS may decrease all-cause mortality, serious adverse events, the number of people who did not receive a liver transplant, and the days of hospitalisation because of the very low-certainty evidence. We are unsure if TIPS, compared with conventional treatment, has better effects on overall morbidity (bacterial peritonitis, encephalopathy, or refractory ascites). TIPS may improve kidney function, but the certainty of evidence is low. The trials included no data on health-related quality of life, non-serious adverse events, and liver function associated with the TIPS placement. We identified one ongoing trial and one study awaiting classification which may contribute to the review when information becomes available.
Trial registration: ClinicalTrials.gov NCT00222014 NCT04315571 NCT05346393.
Copyright © 2024 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
AG: none.
AS: none.
LV: none.
NF: none.
IA: none.
Figures
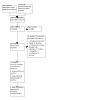


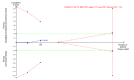











Update of
References
References to studies included in this review
Ginès 2002 {published and unpublished data}
Rössle 2000 {published and unpublished data}
References to studies excluded from this review
Adhoute 2014 {published and unpublished data}
-
- Adhoute X, Castellani P, Penaranda G, Monnet O, Perrier H, Pol B, et al. Does transjugular intrahepatic portosystemic shunt (TIPS) with covered stents modify the natural course of decompensed cirrhosis? Journal of Gastroenterology and Hepatology 2014;29(S3):178. [DOI: 10.1111/jgh.12766_2] - DOI
-
- Adhoute X, Castellani P, Penaranda G, Monnet O, Perrier H, Pol BL, et al. Does transjugular intrahepatic portosystemic shunt (TIPS) with covered stents modify the natural course of decompensed cirrhosis? Hepatology 2014;60:334A. [DOI: 10.1002/hep.27493] - DOI
Alam 1995 {published and unpublished data}7705589
Appenrodt 2009 {published and unpublished data}19794309
-
- Appenrodt B, Zielinski J, Brensing KA, Heller J, Sauerbruch T, Schepke M. Degree of hepatic dysfunction and improvement of renal function predict survival in patients with HRS type I: a retrospective analysis. European Journal of Gastroenterology and Hepatology 2009;21(12):1428-32. [DOI: 10.1097/MEG.0b013e32832ec16a] [PMID: ] - DOI - PubMed
Brensing 1997 {published and unpublished data}9078203
Brensing 2000 {published and unpublished data}
-
- Brensing KA, Textor J, Perz J, Schiedermaier P, Raab P, Strunk H, et al. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut 2000;47(2):288-95. [DOI: 10.1136/gut.47.2.288] [PMID: ] - DOI - PMC - PubMed
Charilaou 2020 {published and unpublished data}32062714
Michl 2000 {published and unpublished data}10912668
-
- Michl P, Gülberg V, Bilzer M, Waggershauser T, Reiser M, Gerbes AL. Transjugular intrahepatic portosystemic shunt for cirrhosis and ascites: effects in patients with organic or functional renal failure. Scandinavian Journal of Gastroenterology 2000;35(6):654-8. [DOI: 10.1080/003655200750023642] [PMID: ] - DOI - PubMed
NCT00222014 {unpublished data only}
-
- NCT00222014. TIPS with coated stents for refractory ascites in patients with cirrhosis. clinicaltrials.gov/ct2/show/NCT00222014 (first received 22 September 2005).
NCT04315571 {unpublished data only}
-
- NCT04315571. Comparing ascites relief in two standard treatments: large volume paracentesis vs. early TIPS using Viatorr controlled expansion stents. clinicaltrials.gov/ct2/show/NCT04315571 (first received 19 March 2020).
Trivedi 2020 {published and unpublished data}31541656
-
- Trivedi P, Biggins S, Crow R, Ryu R, Rochon P. Reduced inpatient mortality with transjugular intrahepatic portosystemic shunt (TIPS) creation in cirrhotic patients admitted with hepatorenal syndrome. Journal of Vascular and Interventional Radiology 2016;27(Suppl 3):S192. [DOI: 10.1016/j.jvir.2015.12.496] - DOI
-
- Trivedi P, Nguyentat M, Brown M, Rochon P, Ryu R, Johnson D. Sex disparity in survival following transjugular intrahepatic portosystemic shunt creation in patients with hepatorenal syndrome. Journal of Vascular and Interventional Radiology 2019;30(3 Suppl):S124. [DOI: 10.1016/j.jvir.2018.12.340] - DOI
-
- Trivedi PS, Brown MA, Rochon PJ, Ryu RK, Johnson DT. Gender disparity in inpatient mortality after transjugular intrahepatic portosystemic shunt creation in patients admitted with hepatorenal syndrome: a nationwide study. Journal of the American College of Radiology 2020;17(2):231-7. [DOI: 10.1016/j.jacr.2019.08.020] [PMID: ] - DOI - PubMed
References to studies awaiting assessment
Junge 2010 {published and unpublished data}
-
- Junge G, Schewior L, Neuhaus P. Hepatorenal syndrome in patients waiting for LTx – efficacy of different plasma volume expansion/vasoconstriction regimens compared to TIPS. Liver Transplantation 2010;16(1):S81. [DOI: 10.1002/lt.22086.] - DOI
-
- Schewior L, Frei U, Neuhaus P, Junge G. Treatment and impact of hepatorenal syndrome for patients on waiting list for liver transplantation. Liver Transplantation 2008;14(S1):S219-20. [DOI: 10.1002/lt.21569] - DOI
References to ongoing studies
NCT05346393 {published and unpublished data}
-
- NCT05346393. HRS-AKI treatment with TIPS in patients with cirrhosis (Liver-HERO). clinicaltrials.gov/ct2/show/NCT05346393 (first posted 26 April 2022).
-
- Ripoll C, Platzer S, Franken P, Aschenbach R, Wienke A, Schuhmacher U. Liver-HERO: hepatorenal syndrome-acute kidney injury (HRS-AKI) treatment with transjugular intrahepatic portosystemic shunt in patients with cirrhosis—a randomized controlled trial. Trials 2023;24:258. [DOI: 10.1186/s13063-023-07261-9] [PMID: ] - DOI - PMC - PubMed
Additional references
Albillos 2005
Alessandria 2002
-
- Alessandria C, Venon WD, Marzano A, Barletti C, Fadda M, Rizzetto M. Renal failure in cirrhotic patients: role of terlipressin in clinical approach to hepatorenal syndrome type 2. European Journal of Gastroenterology and Hepatology 2002;14(12):1363-8. [DOI: 10.1097/00042737-200212000-00013] [PMID: ] - DOI - PubMed
Angeli 2015
-
- Angeli P, Gines P, Wong F, Bernardi M, Boyer T, Gerbes A, et al. International Club of Ascites. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Hepatology 2015;64:531-7. [DOI: 10.1136/gutjnl-2014-308874] [PMID: ] - DOI - PubMed
Arroyo 2008
Bai 2014
Bera 2022
Boutron 2022
-
- Boutron I, Page MJ, Higgins JP, Altman DG, Lundh A, Hróbjartsson A. Chapter 7: Considering bias and conflicts of interest among the included studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Brensing 2000
-
- Brensing KA, Textor J, Perz J, Schiedermaier P, Raab P, Strunk H, et al. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut 2000;4(2):288-95. [DOI: 10.1136/gut.47.2.288] [PMID: ] - DOI - PMC - PubMed
Brok 2008
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive – trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. International Journal of Epidemiology 2009;38(1):287-98. [DOI: 10.1093/ije/dyn188] - DOI - PubMed
Cárdenas 2003
Davenport 2012
Deeks 2022
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
DeMets 1987
-
- DeMets DL. Methods of combining randomised clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341-50. [PMID: ] - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [PMID: ] - PubMed
Duvoux 2002
Egger 1997
Egger 2003
-
- Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technology Assessment 2003;7(1):1-76. [PMID: ] - PubMed
EndNote [Computer program]
-
- EndNote 21. Version 21. London, UK: Clarivate Analytics, 2023. Available at endnote.com.
Gerbes 1998
Ginès 2003
Gluud 2010
Gluud 2012
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 28 September 2023. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guevara 1998
-
- Guevara M, Ginès P, Bandi JC, Gilabert R, Sort P, Jiménez W, et al. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology 1998;28(2):416-22. - PubMed
Guevara 2011
Guyatt 2008
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Research Ed.) 2008;336(7650):924-6. [DOI: 10.1136/bmj.39489.470347.AD] - DOI - PMC - PubMed
Harbord 2006
Hasper 2011
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2022a
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Higgins 2022b
-
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Hollis 1999
ICH‐GCP 2016
-
- International Council for Harmonisation of technical requirements for pharmaceuticals for human use (ICH). ICH Harmonised Guideline. Integrated addendum to ICH E6(R1): guideline for good clinical practice E6(R2). database.ich.org/sites/default/files/E6_R2_Addendum.pdf (accessed 19 July 2023).
Ioannou 2003
Jakobsen 2014
Jalan 1996
-
- Jalan R, Redhead DN, Thomas HW, Henderson N, O'Rourke K, Dillon JF, et al. Mechanisms of changes in renal handling of sodium following transjugular intrahepatic portal systemic stent-shunt (TIPSS). European Journal of Gastroenterology and Hepatology 1996;8(11):1111-6. [PMID: ] - PubMed
Kjaergaard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine 2001;135(11):982-9. - PubMed
Kjaergard 2003
Macaskill 2001
Page 2021a
Page 2021b
Page 2022
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
RevMan Web 2023 [Computer program]
-
- Review Manager Web (RevMan Web). Version 6.2.0. The Cochrane Collaboration, 2023. Available at revman.cochrane.org.
Rössle 2010
Saab 2006
Salerno 2007
Schünemann 2022
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Skagen 2009
Stadlbauer 2008
Sterne 2019
-
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed.) 2019;366:l4898. - PubMed
Testino 2003
-
- Testino G, Ferro C, Sumberaz A, Messa P, Morelli N, Guadagni B, et al. Type-2 hepatorenal syndrome and refractory ascites: role of transjugular intrahepatic portosystemic stent-shunt in eighteen patients with advanced cirrhosis awaiting orthotopic liver transplantation. Hepato-Gastroenterology 2003;50(54):1753-5. [PMID: ] - PubMed
Thorlund 2009
Thorlund 2010
Thorlund 2017
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA); 2nd edition. Copenhagen Trial Unit, 2017. Available from ctu.dk/tsa/learn-more (accessed 19 July 2023).
TSA 2021 [Computer program]
-
- TSA – Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2021. www.ctu.dk/tsa/downloads.aspx.
Wadei 2012
Weissenborn 2019
Wetterslev 2008
Wetterslev 2009
Wetterslev 2017
Whitfield 2009
Wong 1995
-
- Wong F, Sniderman K, Liu P, Allidina Y, Sherman M, Blendis L. Transjugular intrahepatic portosystemic stent shunt: effects on haemodynamics and sodium homeostasis in cirrhosis and refractory ascites. Annals of Internal Medicine 1995;122(11):816-22. [DOI: 10.7326/0003-4819-122-11-199506010-00002] - DOI - PubMed
Wong 2004
World Bank 2023
-
- The World Bank. High income data. data.worldbank.org/country/XD (accessed prior to 9 June 2023).
References to other published versions of this review
Hinojosa‐Azaola 2014
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

