Non-Fourier Bioheat Transfer Analysis in Brain Tissue During Interstitial Laser Ablation: Analysis of Multiple Influential Factors
- PMID: 38236341
- PMCID: PMC11252202
- DOI: 10.1007/s10439-023-03433-5
Non-Fourier Bioheat Transfer Analysis in Brain Tissue During Interstitial Laser Ablation: Analysis of Multiple Influential Factors
Abstract
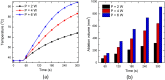
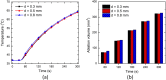
This work presents the dual-phase lag-based non-Fourier bioheat transfer model of brain tissue subjected to interstitial laser ablation. The finite element method has been utilized to predict the brain tissue's temperature distributions and ablation volumes. A sensitivity analysis has been conducted to quantify the effect of variations in the input laser power, treatment time, laser fiber diameter, laser wavelength, and non-Fourier phase lags. Notably, in this work, the temperature-dependent thermal properties of brain tissue have been considered. The developed model has been validated by comparing the temperature obtained from the numerical and ex vivo brain tissue during interstitial laser ablation. The ex vivo brain model has been further extended to in vivo settings by incorporating the blood perfusion effects. The results of the systematic analysis highlight the importance of considering temperature-dependent thermal properties of the brain tissue, non-Fourier behavior, and microvascular perfusion effects in the computational models for accurate predictions of the treatment outcomes during interstitial laser ablation, thereby minimizing the damage to surrounding healthy tissue. The developed model and parametric analysis reported in this study would assist in a more accurate and precise prediction of the temperature distribution, thus allowing to optimize the thermal dosage during laser therapy in the brain.
Keywords: Bioheat transfer; Brain; Laser ablation; Mathematical modeling; Non-Fourier heat transfer; Thermal therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Local thermal non-equilibrium bioheat transfer model for interstitial hyperthermia treatment of tumour cell: A numerical approach.J Therm Biol. 2022 Dec;110:103368. doi: 10.1016/j.jtherbio.2022.103368. Epub 2022 Oct 11. J Therm Biol. 2022. PMID: 36462865
-
Coupled thermo-electro-mechanical models for thermal ablation of biological tissues and heat relaxation time effects.Phys Med Biol. 2019 Dec 13;64(24):245008. doi: 10.1088/1361-6560/ab4cc5. Phys Med Biol. 2019. PMID: 31600740
-
Numerical study of non-Fourier heat conduction in a biolayer spherical living tissue during hyperthermia.J Therm Biol. 2016 Dec;62(Pt B):181-188. doi: 10.1016/j.jtherbio.2016.06.019. Epub 2016 Jul 2. J Therm Biol. 2016. PMID: 27888932
-
Modeling Heat Transfer in Tumors: A Review of Thermal Therapies.Ann Biomed Eng. 2019 Mar;47(3):676-693. doi: 10.1007/s10439-018-02177-x. Epub 2018 Dec 10. Ann Biomed Eng. 2019. PMID: 30536025 Review.
-
Thermophysical and mechanical properties of biological tissues as a function of temperature: a systematic literature review.Int J Hyperthermia. 2022;39(1):297-340. doi: 10.1080/02656736.2022.2028908. Int J Hyperthermia. 2022. PMID: 35129046
References
-
- Pacella, C. M., and G. Mauri. History of laser ablation. In: Image-Guided Laser Ablation. Cham: Springer, 2020, pp. 1–5.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

