Tremelimumab: A Review in Advanced or Unresectable Hepatocellular Carcinoma
- PMID: 38236364
- PMCID: PMC10954993
- DOI: 10.1007/s11523-023-01026-9
Tremelimumab: A Review in Advanced or Unresectable Hepatocellular Carcinoma
Erratum in
-
Correction to: Tremelimumab: A Review in Advanced or Unresectable Hepatocellular Carcinoma.Target Oncol. 2024 May;19(3):481. doi: 10.1007/s11523-024-01053-0. Target Oncol. 2024. PMID: 38507179 Free PMC article. No abstract available.
Abstract
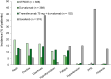
Tremelimumab (tremelimumab-actl; Imjudo®) is a monoclonal antibody and immune checkpoint inhibitor (ICI) that blocks cytotoxic T lymphocyte-associated antigen-4 (CTLA-4). A single, priming dose of intravenous tremelimumab is used in combination with durvalumab, an ICI that blocks programmed cell-death ligand 1, in a regimen known as STRIDE (Single Tremelimumab Regular Interval Durvalumab). STRIDE is approved for the treatment of adults with unresectable hepatocellular carcinoma (HCC) in the USA and Japan and for the first-line treatment of adults with advanced or unresectable HCC in Europe. In the phase III HIMALAYA trial, STRIDE significantly improved overall survival (OS) compared with sorafenib in adults with unresectable HCC and no prior systemic therapy. A higher proportion of STRIDE versus sorafenib recipients had an objective response to treatment. The OS benefit associated with STRIDE was sustained with 4 years' follow-up. STRIDE had a manageable safety profile that differed from that of sorafenib. Grade 3 or 4 treatment-related adverse events occurred in a lower proportion of STRIDE versus sorafenib recipients. Based on the available evidence, tremelimumab used as part of the STRIDE regimen is a valuable first-line agent that expands the treatment options available to patients with advanced or unresectable HCC.
Plain language summary
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and a leading cause of cancer death worldwide. HCC is commonly associated with cirrhosis linked to chronic viral hepatitis and non-alcoholic fatty liver disease. Tremelimumab (tremelimumab-actl; Imjudo®) is a type of immunotherapy that helps the body’s immune system attack HCC cells by binding to and blocking the action of an immune-checkpoint protein called cytotoxic T lymphocyte-associated antigen-4. A single dose of intravenous tremelimumab is used in combination with treatment with durvalumab, in a regimen known as STRIDE (Single Tremelimumab Regular Interval Durvalumab), for adults with unresectable HCC in the USA and Japan and as a first-line treatment for adults with advanced or unresectable HCC in the EU. In patients with unresectable HCC, STRIDE improved overall survival more than sorafenib, including at 4 years’ follow-up. A higher proportion of patients responded to treatment with STRIDE compared with sorafenib. STRIDE had manageable adverse events. Tremelimumab used as part of the STRIDE regimen is a valuable first-line agent that expands the treatment options available to patients with HCC that is advanced or unable to be removed with surgery.
© 2024. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
Nicole L. France and Hannah A. Blair are salaried employees of Adis International Ltd/Springer Nature, and declare no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Figures

References
-
- Asafo-Agyei KO, Samant H. Hepatocellular Carcinoma [updated 2023 June 12] In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; Jan-. 2023. https://www.ncbi.nlm.nih.gov/pubmed/32644603. Accessed 5 Dec 2023.
-
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Hepatocellular carcinoma version 2. 2023. https://www.nccn.org/professionals/physician_gls/pdf/hcc.pdf. Accessed 5 Dec 2023.
-
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. doi: 10.1158/2159-8290.CD-18-0367. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

