Restraining of glycoprotein VI- and integrin α2β1-dependent thrombus formation by platelet PECAM1
- PMID: 38236412
- PMCID: PMC10796532
- DOI: 10.1007/s00018-023-05058-2
Restraining of glycoprotein VI- and integrin α2β1-dependent thrombus formation by platelet PECAM1
Abstract
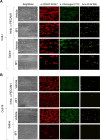
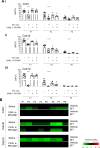
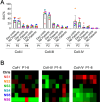
The platelet receptors, glycoprotein VI (GPVI) and integrin α2β1 jointly control collagen-dependent thrombus formation via protein tyrosine kinases. It is unresolved to which extent the ITIM (immunoreceptor tyrosine-based inhibitory motif) receptor PECAM1 and its downstream acting protein tyrosine phosphatase PTPN11 interfere in this process. Here, we hypothesized that integrin α2β1 has a co-regulatory role in the PECAM1- and PTPN11-dependent restraint of thrombus formation. We investigated platelet activation under flow on collagens with a different GPVI dependency and using integrin α2β1 blockage. Blood was obtained from healthy subjects and from patients with Noonan syndrome with a gain-of-function mutation of PTPN11 and variable bleeding phenotype. On collagens with decreasing GPVI activity (types I, III, IV), the surface-dependent inhibition of PECAM1 did not alter thrombus parameters using control blood. Blockage of α2β1 generally reduced thrombus parameters, most effectively on collagen IV. Strikingly, simultaneous inhibition of PECAM1 and α2β1 led to a restoration of thrombus formation, indicating that the suppressing signaling effect of PECAM1 is masked by the platelet-adhesive receptor α2β1. Blood from 4 out of 6 Noonan patients showed subnormal thrombus formation on collagen IV. In these patients, effects of α2β1 blockage were counterbalanced by PECAM1 inhibition to a normal phenotype. In summary, we conclude that the suppression of GPVI-dependent thrombus formation by either PECAM1 or a gain-of-function of PTPN11 can be overruled by α2β1 engagement.
Keywords: Collagens; Microfluidics; Microspots; Noonan syndrome; PTPN11; SHP2.
© 2024. The Author(s).
Conflict of interest statement
J.W.M.H. is a consultant at Synapse Research Institute. The other authors declare no relevant conflict of interest.
Figures




References
-
- Lecut C, Schoolmeester A, Kuijpers MJ, Broers JL, van Zandvoort MA, Vanhoorelbeke K, Deckmyn H, Jandrot-Perrus M, Heemskerk JW. Principal role of glycoprotein VI in a2b1 and aIIbb3 activation during collagen-induced thrombus formation. Arterioscler Thromb Vasc Biol. 2004;24:1727–1733. doi: 10.1161/01.ATV.0000137974.85068.93. - DOI - PubMed
-
- Nagy M, van Geffen JP, Stegner D, Adams DJ, Braun A, de Witt SM, Elvers M, Geer MJ, Kuijpers MJ, Kunzelmann K, Mori J, Oury C, Pircher J, Pleines I, Poole AW, Senis YA, Verdoold R, Weber C, Nieswandt B, Heemskerk JW, Baaten CC. Comparative analysis of microfluidics thrombus formation in multiple genetically modified mice: link to thrombosis and hemostasis. Front Cardiovasc Med. 2019;6:99. doi: 10.3389/fcvm.2019.00099. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

