Design and Synthesis of Dual-Targeting Inhibitors of sEH and HDAC6 for the Treatment of Neuropathic Pain and Lipopolysaccharide-Induced Mortality
- PMID: 38236416
- PMCID: PMC11308793
- DOI: 10.1021/acs.jmedchem.3c02006
Design and Synthesis of Dual-Targeting Inhibitors of sEH and HDAC6 for the Treatment of Neuropathic Pain and Lipopolysaccharide-Induced Mortality
Abstract
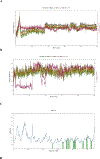
Epoxyeicosatrienoic acids with anti-inflammatory effects are inactivated by soluble epoxide hydrolase (sEH). Both sEH and histone deacetylase 6 (HDAC6) inhibitors are being developed as neuropathic pain relieving agents. Based on the structural similarity, we designed a new group of compounds with inhibition of both HDAC6 and sEH and obtained compound M9. M9 exhibits selective inhibition of HDAC6 over class I HDACs in cells. M9 shows good microsomal stability, moderate plasma protein binding rate, and oral bioavailability. M9 exhibited a strong analgesic effect in vivo, and its analgesic tolerance was better than gabapentin. M9 improved the survival time of mice treated with lipopolysaccharide (LPS) and reversed the levels of inflammatory factors induced by LPS in mouse plasma. M9 represents the first sEH/HDAC6 dual inhibitors with in vivo antineuropathic pain and anti-inflammation.
Conflict of interest statement
The authors declare no competing financial interest conflict.
Figures
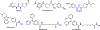








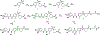


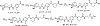
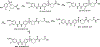


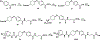

Similar articles
-
Design and Synthesis of sEH/HDAC6 Dual-Targeting Inhibitors for the Treatment of Inflammatory Pain.J Med Chem. 2024 Aug 8;67(15):12887-12911. doi: 10.1021/acs.jmedchem.4c00847. Epub 2024 Jul 21. J Med Chem. 2024. PMID: 39033411 Free PMC article.
-
A multimodal disease modifying approach to treat neuropathic pain--inhibition of soluble epoxide hydrolase (sEH).Drug Discov Today. 2015 Nov;20(11):1382-90. doi: 10.1016/j.drudis.2015.07.017. Epub 2015 Aug 8. Drug Discov Today. 2015. PMID: 26259523 Review.
-
Optimized inhibitors of soluble epoxide hydrolase improve in vitro target residence time and in vivo efficacy.J Med Chem. 2014 Aug 28;57(16):7016-30. doi: 10.1021/jm500694p. Epub 2014 Aug 11. J Med Chem. 2014. PMID: 25079952 Free PMC article.
-
Evaluation of the Therapeutic Potential of Sulfonyl Urea Derivatives as Soluble Epoxide Hydrolase (sEH) Inhibitors.Molecules. 2024 Jun 26;29(13):3036. doi: 10.3390/molecules29133036. Molecules. 2024. PMID: 38998987 Free PMC article.
-
Novel Selective Histone Deacetylase 6 (HDAC6) Inhibitors: A Patent Review (2016-2019).Recent Pat Anticancer Drug Discov. 2020;15(1):32-48. doi: 10.2174/1574892815666200217125419. Recent Pat Anticancer Drug Discov. 2020. PMID: 32065106 Review.
Cited by
-
System-wide targeted analysis of oxylipins and lipoproteins in chronic peripheral neuropathic pain-an explorative study.Pain Rep. 2025 Jul 2;10(4):e1305. doi: 10.1097/PR9.0000000000001305. eCollection 2025 Aug. Pain Rep. 2025. PMID: 40612405 Free PMC article.
References
-
- Treede RD; Jensen TS; Campbell JN; Cruccu G; Dostrovsky JO; Griffin JW; Hansson P; Hughes R; Nurmikko T; Serra J Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008, 70, 1630–1635. - PubMed
-
- Bannister K; Sachau J; Baron R; Dickenson AH Neuropathic pain: mechanism-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 257–274. - PubMed
-
- Finnerup NB; Kuner R; Jensen TS Neuropathic pain: from mechanisms to treatment. Physiol. Rev. 2021, 101, 259–301. - PubMed
-
- Bair MJ; Robinson RL; Katon W; Kroenke K Depression and pain comorbidity: a literature review. Arch. Intern. Med. 2003, 163, 2433–2445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

