Evaluation of the introduction of novel potassium binders in routine care; the Stockholm CREAtinine measurements (SCREAM) project
- PMID: 38236474
- PMCID: PMC11239771
- DOI: 10.1007/s40620-023-01860-0
Evaluation of the introduction of novel potassium binders in routine care; the Stockholm CREAtinine measurements (SCREAM) project
Abstract
Background: The pharmacological management of hyperkalemia traditionally considered calcium or sodium polystyrene sulfonate and, since recently, the novel binders patiromer and sodium zirconium cyclosilicate. We evaluated their patterns of use, duration of treatment and relative effectiveness/safety in Swedish routine care.
Methods: Observational study of adults initiating therapy with sodium polystyrene sulfonate or a novel binder (sodium zirconium cyclosilicate or patiromer) in Stockholm 2019-2021. We quantified treatment duration by repeated dispensations, compared mean achieved potassium concentration within 60 days, and potential adverse events between treatments.
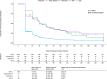
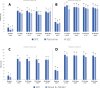
Results: A total of 1879 adults started treatment with sodium polystyrene sulfonate, and 147 with novel binders (n = 41 patiromer and n = 106 sodium zirconium cyclosilicate). Potassium at baseline for all treatments was 5.7 mmol/L. Sodium polystyrene sulfonate patients stayed on treatment a mean of 61 days (14% filled ≥3 consecutive prescriptions) compared to 109 days on treatment (49% filled ≥3 prescriptions) for novel binders. After 15 days of treatment, potassium similarly decreased to 4.6 (SD 0.6) and 4.8 (SD 0.6) mmol/L in the sodium polystyrene sulfonate and novel binder groups, respectively, and was maintained over the 60 days post-treatment. In multivariable regression, the odds ratio for novel binders (vs sodium polystyrene sulfonate) in reaching potassium ≤ 5.0 mmol/L after 15 days was 0.65 (95% CI 0.38-1.10) and after 60 days 0.89 (95% CI 0.45-1.76). Hypocalcemia, hypokalemia, and initiation of anti-diarrheal/constipation medications were the most-commonly detected adverse events. In multivariable analyses, the OR for these events did not differ between groups.
Conclusion: We observed similar short-term effectiveness and safety for all potassium binders. However, treatment duration was longer for novel binders than for sodium polystyrene sulfonate.
Keywords: Hyperkalemia; Patiromer; SCREAM; Sodium polystyrene sulfonate; Sodium zirconium cyclosilicate.
© 2024. The Author(s).
Conflict of interest statement
JJC has conducted studies for which Karolinska Institutet has received support from AstraZeneca (manufacturer of sodium zirconium cyclosilicate), ViforPharma (manufacturer of patiromer), Novonordisk, Astellas, MSD, GSK, Boehringer Ingelheim and Amgen; He also reports receiving lecture fees from Baxter, Fresenius Kabi, AstraZeneca, Astellas, GSK and Abbott; and participation in advisory boards for Astrazeneca, Nestle and Bayer; ME reports payment for lectures (Astellas pharma, Vifor pharma, Astra Zeneca, Baxter healthcare and Fresenius Medical care) and attendance in advisory boards (Astellas pharma, Vifor pharma, Astra Zeneca). The rest of the authors have no conflict of interest to report.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

