An Engineered Bionic Nanoparticle Sponge as a Cytokine Trap and Reactive Oxygen Species Scavenger to Relieve Disc Degeneration and Discogenic Pain
- PMID: 38237054
- PMCID: PMC10832058
- DOI: 10.1021/acsnano.3c08097
An Engineered Bionic Nanoparticle Sponge as a Cytokine Trap and Reactive Oxygen Species Scavenger to Relieve Disc Degeneration and Discogenic Pain
Abstract
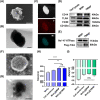
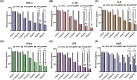
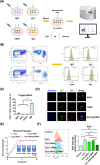
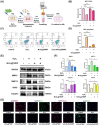
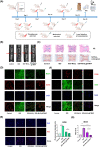
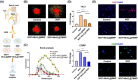
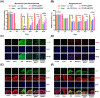
The progressive worsening of disc degeneration and related nonspecific back pain are prominent clinical issues that cause a tremendous economic burden. Activation of reactive oxygen species (ROS) related inflammation is a primary pathophysiologic change in degenerative disc lesions. This pathological state is associated with M1 macrophages, apoptosis of nucleus pulposus cells (NPC), and the ingrowth of pain-related sensory nerves. To address the pathological issues of disc degeneration and discogenic pain, we developed MnO2@TMNP, a nanomaterial that encapsulated MnO2 nanoparticles with a TrkA-overexpressed macrophage cell membrane (TMNP). Consequently, this engineered nanomaterial showed high efficiency in binding various inflammatory factors and nerve growth factors, which inhibited inflammation-induced NPC apoptosis, matrix degradation, and nerve ingrowth. Furthermore, the macrophage cell membrane provided specific targeting to macrophages for the delivery of MnO2 nanoparticles. MnO2 nanoparticles in macrophages effectively scavenged intracellular ROS and prevented M1 polarization. Supportively, we found that MnO2@TMNP prevented disc inflammation and promoted matrix regeneration, leading to downregulated disc degenerative grades in the rat injured disc model. Both mechanical and thermal hyperalgesia were alleviated by MnO2@TMNP, which was attributed to the reduced calcitonin gene-related peptide (CGRP) and substance P expression in the dorsal root ganglion and the downregulated Glial Fibrillary Acidic Protein (GFAP) and Fos Proto-Oncogene (c-FOS) signaling in the spinal cord. We confirmed that the MnO2@TMNP nanomaterial alleviated the inflammatory immune microenvironment of intervertebral discs and the progression of disc degeneration, resulting in relieved discogenic pain.
Keywords: ROS scavenger; biomembrane-coated nanoparticles; cytokine trap; disc degeneration; discogenic pain.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Pan H.; Strickland A.; Madhu V.; Johnson Z. I.; Chand S. N.; Brody J. R.; Fertala A.; Zheng Z.; Shapiro I. M.; Risbud M. V. RNA binding protein HuR regulates extracellular matrix gene expression and pH homeostasis independent of controlling HIF-1α signaling in nucleus pulposus cells. Matrix biology: journal of the International Society for Matrix Biology 2019, 77, 23–40. 10.1016/j.matbio.2018.08.003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

