Overcoming a Radical Polarity Mismatch in Strain-Release Pentafluorosulfanylation of [1.1.0]Bicyclobutanes: An Entryway to Sulfone- and Carbonyl-Containing SF5-Cyclobutanes
- PMID: 38237059
- PMCID: PMC11045327
- DOI: 10.1002/anie.202319930
Overcoming a Radical Polarity Mismatch in Strain-Release Pentafluorosulfanylation of [1.1.0]Bicyclobutanes: An Entryway to Sulfone- and Carbonyl-Containing SF5-Cyclobutanes
Abstract
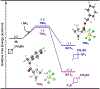
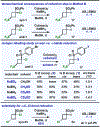
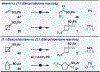
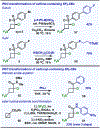
The first assortment of achiral pentafluorosulfanylated cyclobutanes (SF5-CBs) are now synthetically accessible through strain-release functionalization of [1.1.0]bicyclobutanes (BCBs) using SF5Cl. Methods for both chloropentafluorosulfanylation and hydropentafluorosulfanylation of sulfone-based BCBs are detailed herein, as well as proof-of-concept that the logic extends to tetrafluoro(aryl)sulfanylation, tetrafluoro(trifluoromethyl)sulfanylation, and three-component pentafluorosulfanylation reactions. The methods presented enable isolation of both syn and anti isomers of SF5-CBs, but we also demonstrate that this innate selectivity can be overridden in chloropentafluorosulfanylation; that is, an anti-stereoselective variant of SF5Cl addition across sulfone-based BCBs can be achieved by using inexpensive copper salt additives. Considering the SF5 group and CBs have been employed individually as nonclassical bioisosteres, structural aspects of these unique SF5-CB "hybrid isosteres" were then contextualized using SC-XRD. From a mechanistic standpoint, chloropentafluorosulfanylation ostensibly proceeds through a curious polarity mismatch addition of electrophilic SF5 radicals to the electrophilic sites of the BCBs. Upon examining carbonyl-containing BCBs, we also observed rare instances whereby radical addition to the 1-position of a BCB occurs. The nature of the key C(sp3)-SF5 bond formation step - among other mechanistic features of the methods we disclose - was investigated experimentally and with DFT calculations. Lastly, we demonstrate compatibility of SF5-CBs with various downstream functionalizations.
Keywords: cyclobutanes; hybrid isosteres; pentafluorosulfanylation; radical reactions; strain-release functionalization.
© 2024 Wiley‐VCH GmbH.
Figures
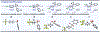

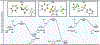
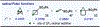




References
-
- Altomonte S, Zanda M, J. Fluorine Chem 2012, 143, 57–93;
- Savoie PR, Welch JT, Chem. Rev 2015, 115, 1130–1190; - PubMed
- Gillis EP, Eastman KJ, Hill MD, Donnelly DJ, Meanwell NA, J. Med. Chem 2015, 58, 8315–8359; - PubMed
- Sani M, Zanda M, Synthesis 2022, 54, 4184–4209;
- Magre M, Ni S, Cornella J, Angew. Chem. Int. Ed 2022, 61, e202200904; - PMC - PubMed
- Haufe G, Tetrahedron, 2022, 109, 132656–132691.
-
- For recent applications of TCICA/KF in aryl-SF5 compound synthesis, see: a) Pitts CR, Bornemann D, Liebing P, Santschi N, Togni A, Angew. Chem. Int. Ed 2019, 58, 1950–1954; - PubMed
- Wang L, Cornella J, Angew. Chem. Int. Ed 2020, 59, 23510–23515; - PMC - PubMed
- Wang L, Ni S, Cornella J, Synthesis 2021, 53, 4308–4312.
-
- For other recent applications of TCICA/KF in oxidative fluorination of heteroatoms, see: a) Bornemann D, Pitts CR, Ziegler CJ, Pietrasiak E, Trapp N, Santschi N, Togni A, Angew. Chem. Int. Ed 2019, 58, 12604–12608; - PubMed
- Brüning F, Pitts CR, Kalim J, Bornemann D, Ghiazza C, de Montmillon J, Trapp N, Billard T, Togni A, Angew. Chem. Int. Ed 2019, 58, 18937–18941; - PubMed
- Häfliger J, Pitts CR, Bornemann D, Käser R, Santschi N, Charpentier J, Otth E, Trapp N, Verel R, Lüthi HP, Togni A, Chem. Sci 2019, 10, 7251–7259; - PMC - PubMed
- Bornemann D, Brüning F, Bartalucci N, Wettstein L, Pitts CR, Helv. Chim. Acta 2021, 104, e2000218;
- Ragan AN, Kraemer Y, Kong W-Y, Prasad S, Tantillo DJ, Pitts CR, Angew. Chem. Int. Ed 2022, 61, e202208046; - PubMed
- Wegener D, Hoffmann KF, Pérez-Bitrián A, Bayindir I, Hadi AN, Wiesner A, Riedel S, Chem. Commun 2022, 58, 9694–9697. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

