von Willebrand factor/factor VIII concentrate (Wilate) prophylaxis in children and adults with von Willebrand disease
- PMID: 38237075
- PMCID: PMC10950830
- DOI: 10.1182/bloodadvances.2023011742
von Willebrand factor/factor VIII concentrate (Wilate) prophylaxis in children and adults with von Willebrand disease
Abstract
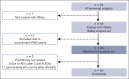
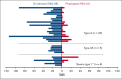
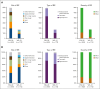
Long-term prophylaxis with a von Willebrand factor (VWF) concentrate is recommended in patients with von Willebrand disease (VWD) who have a history of severe and frequent bleeds. However, data from prospective studies are scarce. WIL-31, a prospective, noncontrolled, international phase 3 trial, investigated the efficacy and safety of Wilate prophylaxis in severe patients with VWD. Male and female patients 6 years or older with VWD types 1, 2 (except 2N), or 3 who had completed a prospective, 6-month, on-demand, run-in study (WIL-29) were eligible to receive Wilate prophylaxis for 12 months. At baseline, patients (n = 33) had a median age of 18 years. Six (18%) patients had severe type 1, 5 (15%) had type 2, and 22 (67%) had type 3 VWD. The primary end point of a >50% reduction in mean total annualized bleeding rate (TABR) with Wilate prophylaxis vs prior on-demand treatment was met; mean TABR during prophylaxis was 5.2, representing an 84.4% reduction. The bleeding reduction was consistent across age, sex, and VWD types. The mean spontaneous ABR was 3.2, representing an 86.9% reduction vs on-demand treatment. During prophylaxis, 10 (30.3%) patients had 0 bleeding events and 15 (45.5%) patients had 0 spontaneous bleeding events. Of 173 BEs, 84.4% were minor and 69.9% treated. No serious adverse events related to study treatment and no thrombotic events were recorded. Overall, WIL-31 showed that Wilate prophylaxis was efficacious and well-tolerated in pediatric and adult patients with VWD of all types. The WIL-29 and WIL-31 trials were registered at www.ClinicalTrials.gov as #NCT04053699 and #NCT04052698, respectively.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: R.F.S. Jr has received research funding from Octapharma and Takeda, honoraria for consulting and advisory boards from Octapharma, Takeda, Laboratoire français du Fractionnement et des Biotechnologies (LFB), Sanofi, SOBI, Novo Nordisk, Bayer, Pfizer, BioMarin, Vega, Guardian Therapeutics, payment for expert testimony from Sanofi; has participated on a data safety monitoring board or advisory board for Uniqure; and has had a role as American Thrombosis and Hemostasis Network board member, International Society on Thrombosis and Haemostasis (ISTH) Chair, Hemophilia Federation of America medical adviser, and with the Medical and Scientific Advisory Council of the National Hemophilia Foundation. A.B. has received research funding from Octapharma, honoraria from Amgen, AstraZeneca, Bayer, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, SOBI, Swixx, and Takeda and travel support from SOBI, Novo Nordisk, Takeda; has participated on a data safety monitoring board or advisory board for SOBI, Takeda, Bayer, Swixx, AstraZeneca, and has had a role in the European Association for Haemophilia and Allied Disorders and ISTH. L.D. has participated as a clinical trial investigator for Octapharma. A.I. has received consultancy and research funding from Novartis, Global Blood Therapeutics/Pfizer, Roche, Forma/Novo Nordisk, Vifor, and research funding from Agios and Octapharma. C.K. has received consulting fees from Novo Nordisk Hungaria, Takeda Pharma, and H-W-H; honoraria from Novo Nordisk Hungaria, Takeda Pharma, SOBI, Roche Hungary, and CSL Behring; and travel support from Novo Nordisk Hungaria, SOBI, and CSL Behring. Z.B. has received consulting fees, honoraria, and travel support from Novo Nordisk and Takeda. T.L. has received grant support and speakers fees from Octapharma. L.N. has received consulting fees from Octapharma, CSL Behring, Novo Nordisk, SOBI, and Takeda; honoraria from Octapharma, Takeda, Novo Nordisk, and SOBI; and received travel support from Octapharma, Novo Nordisk, and Takeda. A.T.T. has received grant support and consulting fees and consulting fees from Novartis, Bristol Myers Squibb, Vifor, Agios, and Pharmacosmos. S.W. is an employee of Octapharma USA. S.K is an employee of Octapharma AG. C.D.K. has received presentation and speaker fees from Octapharma, CSL Behring, and LFB and support for travel and attending meetings from Octapharma, CSL Behring, and LFB. The remaining authors declare no competing financial interests.
Figures



References
-
- Leebeek FWG, Eikenboom JCJ. von Willebrand's disease. N Engl J Med. 2016;375(21):2067–2080. - PubMed
-
- Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(suppl 6):1–158. - PubMed
-
- Holm E, Abshire TC, Bowen J, et al. Changes in bleeding patterns in von Willebrand disease after institution of long-term replacement therapy: results from the von Willebrand Disease Prophylaxis Network. Blood Coagul Fibrinolysis. 2015;26(4):383–388. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

