Restoration of the ER stress response protein TDAG51 in hepatocytes mitigates NAFLD in mice
- PMID: 38237682
- PMCID: PMC10875272
- DOI: 10.1016/j.jbc.2024.105655
Restoration of the ER stress response protein TDAG51 in hepatocytes mitigates NAFLD in mice
Abstract
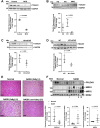
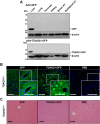
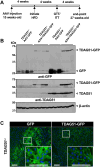
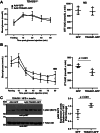
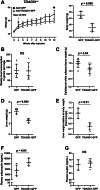
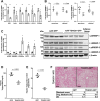
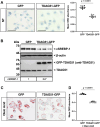
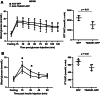
Endoplasmic reticulum stress is associated with insulin resistance and the development of nonalcoholic fatty liver disease. Deficiency of the endoplasmic reticulum stress response T-cell death-associated gene 51 (TDAG51) (TDAG51-/-) in mice promotes the development of high-fat diet (HFD)-induced obesity, fatty liver, and hepatic insulin resistance. However, whether this effect is due specifically to hepatic TDAG51 deficiency is unknown. Here, we report that hepatic TDAG51 protein levels are consistently reduced in multiple mouse models of liver steatosis and injury as well as in liver biopsies from patients with liver disease compared to normal controls. Delivery of a liver-specific adeno-associated virus (AAV) increased hepatic expression of a TDAG51-GFP fusion protein in WT, TDAG51-/-, and leptin-deficient (ob/ob) mice. Restoration of hepatic TDAG51 protein was sufficient to increase insulin sensitivity while reducing body weight and fatty liver in HFD fed TDAG51-/- mice and in ob/ob mice. TDAG51-/- mice expressing ectopic TDAG51 display improved Akt (Ser473) phosphorylation, post-insulin stimulation. HFD-fed TDAG51-/- mice treated with AAV-TDAG51-GFP displayed reduced lipogenic gene expression, increased beta-oxidation and lowered hepatic and serum triglycerides, findings consistent with reduced liver weight. Further, AAV-TDAG51-GFP-treated TDAG51-/- mice exhibited reduced hepatic precursor and cleaved sterol regulatory-element binding proteins (SREBP-1 and SREBP-2). In vitro studies confirmed the lipid-lowering effect of TDAG51 overexpression in oleic acid-treated Huh7 cells. These studies suggest that maintaining hepatic TDAG51 protein levels represents a viable therapeutic approach for the treatment of obesity and insulin resistance associated with nonalcoholic fatty liver disease.
Keywords: hepatocyte; insulin resistance; lipid metabolism; liver; obesity; triglyceride.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Powell E.E., Cooksley W.G., Hanson R., Searle J., Halliday J.W., Powell L.W. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. - PubMed
-
- Matteoni C.A., Younossi Z.M., Gramlich T., Boparai N., Liu Y.C., McCullough A.J. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. - PubMed
-
- Marchesini G., Brizi M., Morselli-Labate A.M., Bianchi G., Bugianesi E., McCullough A.J., et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. 1999;107:450–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

