RvD1 improves resident alveolar macrophage self-renewal via the ALX/MAPK14/S100A8/A9 pathway in acute respiratory distress syndrome
- PMID: 38237770
- PMCID: PMC11725153
- DOI: 10.1016/j.jare.2024.01.017
RvD1 improves resident alveolar macrophage self-renewal via the ALX/MAPK14/S100A8/A9 pathway in acute respiratory distress syndrome
Abstract
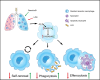
Introduction: Acute respiratory distress syndrome (ARDS) is a pulmonary inflammatory process primarily caused by sepsis. The resolution of inflammation is an active process involving the endogenous biosynthesis of specialized pro-resolving mediators, including resolvin D1 (RvD1). Resident alveolar macrophages (RAMs) maintain pulmonary homeostasis and play a key role in the resolution phase. However, the role of RAMs in promoting the resolution of inflammation by RvD1 is unclear.
Objectives: Here, we investigated the mechanisms of RvD1 on regulating RAMs to promote the resolution of ARDS.
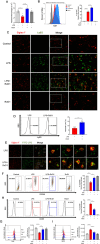
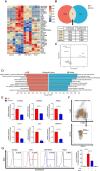
Methods: Mice were administered lipopolysaccharide and/or Escherichia coli via aerosol inhalation to establish a self-limited ARDS model. Then, RvD1 was administered at the peak inflammatory response. RAMs self-renewal was measured by flow cytometry, RAM phagocytosis was measured by two-photon fluorescence imaging. In addition, plasma was collected from intensive care unit patients on days 0-2, 3-5, and 6-9 to measure RvD1 and S100A8/A9 levels using triple quadrupole/linear ion trap mass spectrometry.
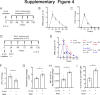
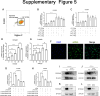
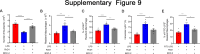
Results: RAMs were found to play a pivotal role in resolving inflammation during ARDS, and RvD1 enhanced RAM proliferation and phagocytosis, which was abrogated by a lipoxin A4 receptor (ALX, RvD1 receptor) inhibitor. Both primary RAMs transfected with rS100A8/A9 and/or S100A8/A9 siRNA and S100A9-/- mice (also deficient in S100A8 function) showed higher turnover and phagocytic function, indicating that RvD1 exerted its effects on RAMs by inhibiting S100A8/A9 production in the resolution phase. RvD1 reduced S100A8/A9 and its upstream MAPK14 levels in vivo and in vitro. Finally, in the patients, RvD1 levels were lower, but S100A8/A9 levels were higher.
Conclusions: We propose that RvD1 improved RAM self-renewal and phagocytosis via the ALX/MAPK14/S100A8/A9 signaling pathway. Plasma RvD1 and S100A8/A9 levels were negatively correlated, and associated with the outcome of sepsis-induced ARDS.
Keywords: Acute respiratory distress syndrome; Resident alveolar macrophages; Resolution of inflammation; Resolvin D1; S100A8/A9.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










References
-
- Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

