Developmental exposure to pesticides that disrupt retinoic acid signaling causes persistent retinoid and behavioral dysfunction in zebrafish
- PMID: 38237923
- PMCID: PMC12104507
- DOI: 10.1093/toxsci/kfae001
Developmental exposure to pesticides that disrupt retinoic acid signaling causes persistent retinoid and behavioral dysfunction in zebrafish
Abstract
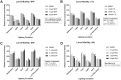
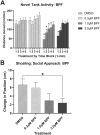
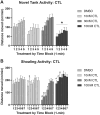
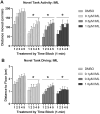
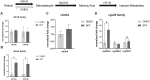
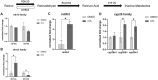
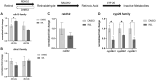
Early developmental exposure to environmental toxicants may play a role in the risk for developing autism. A variety of pesticides have direct effects on retinoic acid (RA) signaling and as RA signaling has important roles in neurodevelopment, such compounds may cause developmental neurotoxicity through an overlapping adverse outcome pathway. It is hypothesized that a pesticide's embryonic effects on retinoid function may correspond with neurobehavioral disruption later in development. In the current studies, we determined the effects of RA-acting pesticides on neurobehavioral development in zebrafish. Buprofezin and imazalil caused generalized hypoactivity in the larval motility test, whereas chlorothalonil and endosulfan I led to selective hypoactivity and hyperactivity, respectively. With buprofezin, chlorothalonil, and imazalil, hypoactivity and/or novel anxiety-like behaviors persisted in adulthood and buprofezin additionally decreased social attraction responses in adulthood. Endosulfan I did not produce significant adult behavioral effects. Using qPCR analyses of adult brain tissue, we observed treatment-induced alterations in RA synthesis or catabolic genes, indicating persistent changes in RA homeostasis. These changes were compound-specific, with respect to expression directionality, and potential patterns of homeostatic disruption. Results suggest the likely persistence of disruptions in RA signaling well into adulthood and may represent compensatory mechanisms following early life stage exposures. This study demonstrates that early developmental exposure to environmental toxicants that interfere with RA signaling causes short as well as long-term behavioral disruption in a well-established zebrafish behavioral model and expand upon the meaning of the RA adverse outcome pathway, indicating that observed effects likely correspond with the nature of underlying homeostatic effects.
Keywords: development; neurobehavioral toxicology; retinoic acid receptors; zebrafish.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Ballard M. S., Sun M., Ko J. (2012). Vitamin a, folate, and choline as a possible preventive intervention to fetal alcohol syndrome. Med. Hypotheses. 78, 489–493. - PubMed
-
- Bedois A. M., Parker H. J., Krumlauf R. (2021). Retinoic acid signaling in vertebrate hindbrain segmentation: Evolution and diversification. Diversity 13, 398.
-
- Chaliha D., Albrecht M., Vaccarezza M., Takechi R., Lam V., Al-Salami H., Mamo J. (2020). A systematic review of the valproic-acid-induced rodent model of autism. Dev. Neurosci. 42, 12–48. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

