Characterization of tRNA splicing enzymes RNA ligase and tRNA 2'-phosphotransferase from the pathogenic fungi Mucorales
- PMID: 38238085
- PMCID: PMC10946426
- DOI: 10.1261/rna.079911.123
Characterization of tRNA splicing enzymes RNA ligase and tRNA 2'-phosphotransferase from the pathogenic fungi Mucorales
Abstract
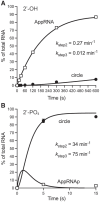
Fungal Trl1 is an essential trifunctional tRNA splicing enzyme that heals and seals tRNA exons with 2',3'-cyclic-PO4 and 5'-OH ends. Trl1 is composed of C-terminal cyclic phosphodiesterase and central polynucleotide kinase end-healing domains that generate the 3'-OH,2'-PO4 and 5'-PO4 termini required for sealing by an N-terminal ATP-dependent ligase domain. Trl1 enzymes are present in many human fungal pathogens and are promising targets for antifungal drug discovery because their domain structures and biochemical mechanisms are unique compared to the mammalian RtcB-type tRNA splicing enzyme. Here we report that Mucorales species (deemed high-priority human pathogens by WHO) elaborate a noncanonical tRNA splicing apparatus in which a monofunctional RNA ligase enzyme is encoded separately from any end-healing enzymes. We show that Mucor circinelloides RNA ligase (MciRNL) is active in tRNA splicing in vivo in budding yeast in lieu of the Trl1 ligase domain. Biochemical and kinetic characterization of recombinant MciRNL underscores its requirement for a 2'-PO4 terminus in the end-joining reaction, whereby the 2'-PO4 enhances the rates of RNA 5'-adenylylation (step 2) and phosphodiester synthesis (step 3) by ∼125-fold and ∼6200-fold, respectively. In the canonical fungal tRNA splicing pathway, the splice junction 2'-PO4 installed by RNA ligase is removed by a dedicated NAD+-dependent RNA 2'-phosphotransferase Tpt1. Here we identify and affirm by genetic complementation in yeast the biological activity of Tpt1 orthologs from three Mucorales species. Recombinant M. circinelloides Tpt1 has vigorous NAD+-dependent RNA 2'-phosphotransferase activity in vitro.
Keywords: fungal pathogen; tRNA 2′-phosphotransferase; tRNA ligase; tRNA splicing.
© 2024 Ghosh et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures








Similar articles
-
Identification, characterization, and structure of a tRNA splicing enzyme RNA 5'-OH kinase from the pathogenic fungi Mucorales.RNA. 2024 Nov 18;30(12):1674-1685. doi: 10.1261/rna.080247.124. RNA. 2024. PMID: 39357987
-
Characterization of the tRNA ligases of pathogenic fungi Aspergillus fumigatus and Coccidioides immitis.RNA. 2016 Oct;22(10):1500-9. doi: 10.1261/rna.057455.116. Epub 2016 Aug 4. RNA. 2016. PMID: 27492257 Free PMC article.
-
Structure-function analysis of the kinase-CPD domain of yeast tRNA ligase (Trl1) and requirements for complementation of tRNA splicing by a plant Trl1 homolog.Nucleic Acids Res. 2006 Jan 20;34(2):517-27. doi: 10.1093/nar/gkj441. Print 2006. Nucleic Acids Res. 2006. PMID: 16428247 Free PMC article.
-
RNA Repair: Hiding in Plain Sight.Annu Rev Genet. 2023 Nov 27;57:461-489. doi: 10.1146/annurev-genet-071719-021856. Epub 2023 Sep 18. Annu Rev Genet. 2023. PMID: 37722686 Review.
-
Insights into the structure and function of the RNA ligase RtcB.Cell Mol Life Sci. 2023 Nov 7;80(12):352. doi: 10.1007/s00018-023-05001-5. Cell Mol Life Sci. 2023. PMID: 37935993 Free PMC article. Review.
Cited by
-
Identification, characterization, and structure of a tRNA splicing enzyme RNA 5'-OH kinase from the pathogenic fungi Mucorales.RNA. 2024 Nov 18;30(12):1674-1685. doi: 10.1261/rna.080247.124. RNA. 2024. PMID: 39357987
-
Kinetic and structural insights into the requirement of fungal tRNA ligase for a 2'-phosphate end.RNA. 2024 Sep 16;30(10):1306-1314. doi: 10.1261/rna.080120.124. RNA. 2024. PMID: 39013577
-
Chemical synthesis of 2″OMeNAD+ and its deployment as an RNA 2'-phosphotransferase (Tpt1) 'poison' that traps the enzyme on its abortive RNA-2'-PO4-(ADP-2″OMe-ribose) reaction intermediate.Nucleic Acids Res. 2024 Sep 23;52(17):10533-10542. doi: 10.1093/nar/gkae695. Nucleic Acids Res. 2024. PMID: 39162230 Free PMC article.
References
-
- Banerjee A, Goldgur Y, Schwer B, Shuman S. 2019b. Atomic structures of the RNA end-healing 5′-OH kinase and 2′,3′-cyclic phosphodiesterase domains of fungal tRNA ligase: conformational switches in the kinase upon binding of the GTP phosphate donor. Nucleic Acids Res 47: 11826–11838. 10.1093/nar/gkz1049 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
