Skeletal editing of pyridines through atom-pair swap from CN to CC
- PMID: 38238464
- PMCID: PMC11087273
- DOI: 10.1038/s41557-023-01428-2
Skeletal editing of pyridines through atom-pair swap from CN to CC
Abstract
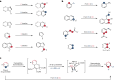
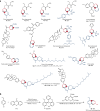
Skeletal editing is a straightforward synthetic strategy for precise substitution or rearrangement of atoms in core ring structures of complex molecules; it enables quick diversification of compounds that is not possible by applying peripheral editing strategies. Previously reported skeletal editing of common arenes mainly relies on carbene- or nitrene-type insertion reactions or rearrangements. Although powerful, efficient and applicable to late-stage heteroarene core structure modification, these strategies cannot be used for skeletal editing of pyridines. Here we report the direct skeletal editing of pyridines through atom-pair swap from CN to CC to generate benzenes and naphthalenes in a modular fashion. Specifically, we use sequential dearomatization, cycloaddition and rearomatizing retrocycloaddition reactions in a one-pot sequence to transform the parent pyridines into benzenes and naphthalenes bearing diversified substituents at specific sites, as defined by the cycloaddition reaction components. Applications to late-stage skeletal diversification of pyridine cores in several drugs are demonstrated.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Skeletal Editing through Cycloaddition and Subsequent Cycloreversion Reactions.Acc Chem Res. 2025 Feb 18;58(4):647-658. doi: 10.1021/acs.accounts.4c00813. Epub 2025 Jan 28. Acc Chem Res. 2025. PMID: 39875197
-
Triftosylhydrazone in Single-Atom Skeletal Editing.Acc Chem Res. 2025 Jan 7;58(1):130-149. doi: 10.1021/acs.accounts.4c00709. Epub 2024 Dec 16. Acc Chem Res. 2025. PMID: 39680057
-
Switchable skeletal editing of quinolines enabled by cyclizative sequential rearrangements.Nat Chem. 2025 Jun;17(6):952-960. doi: 10.1038/s41557-025-01793-0. Epub 2025 Apr 7. Nat Chem. 2025. PMID: 40195435
-
Skeletal Editing of (Hetero)Arenes Using Carbenes.Chemistry. 2023 Jul 26;29(42):e202301227. doi: 10.1002/chem.202301227. Epub 2023 Jul 3. Chemistry. 2023. PMID: 37230933 Review.
-
Skeletal Editing by Hypervalent Iodine Mediated Nitrogen Insertion.Chemistry. 2024 Oct 8;30(56):e202401993. doi: 10.1002/chem.202401993. Epub 2024 Sep 17. Chemistry. 2024. PMID: 39046292 Review.
Cited by
-
Electrochemical meta-C-H sulfonylation of pyridines with nucleophilic sulfinates.Nat Commun. 2024 Aug 28;15(1):7428. doi: 10.1038/s41467-024-50644-y. Nat Commun. 2024. PMID: 39198391 Free PMC article.
-
Selective nitrogen insertion into aryl alkanes.Nat Commun. 2024 Jul 17;15(1):6016. doi: 10.1038/s41467-024-50383-0. Nat Commun. 2024. PMID: 39019881 Free PMC article.
-
Carbon-nitrogen transmutation in polycyclic arenol skeletons to access N-heteroarenes.Nat Commun. 2024 May 4;15(1):3772. doi: 10.1038/s41467-024-48265-6. Nat Commun. 2024. PMID: 38704373 Free PMC article.
-
Synthesis of naphthalene derivatives via nitrogen-to-carbon transmutation of isoquinolines.Sci Adv. 2025 Jan 31;11(5):eads5928. doi: 10.1126/sciadv.ads5928. Epub 2025 Jan 29. Sci Adv. 2025. PMID: 39879292 Free PMC article.
-
Skeletal editing of pyridines to aryldialdehydes.Nat Commun. 2025 Aug 4;16(1):7133. doi: 10.1038/s41467-025-62627-8. Nat Commun. 2025. PMID: 40759905 Free PMC article.
References
-
- Kallitsis JK, Geormezi M, Neophytides SG. Polymer electrolyte membranes for high-temperature fuel cells based on aromatic polyethers bearing pyridine units. Polym. Int. 2009;58:1226–1233. doi: 10.1002/pi.2661. - DOI
-
- Zhou F-Y, Jiao L. Recent developments in transition-metal-free functionalization and derivatization reactions of pyridines. Synlett. 2021;32:159–178. doi: 10.1055/s-0040-1706552. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

