Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial
- PMID: 38238616
- PMCID: PMC10957466
- DOI: 10.1038/s41591-023-02785-8
Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial
Abstract
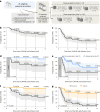
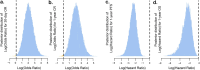
There is a pressing need for allogeneic chimeric antigen receptor (CAR)-immune cell therapies that are safe, effective and affordable. We conducted a phase 1/2 trial of cord blood-derived natural killer (NK) cells expressing anti-CD19 chimeric antigen receptor and interleukin-15 (CAR19/IL-15) in 37 patients with CD19+ B cell malignancies. The primary objectives were safety and efficacy, defined as day 30 overall response (OR). Secondary objectives included day 100 response, progression-free survival, overall survival and CAR19/IL-15 NK cell persistence. No notable toxicities such as cytokine release syndrome, neurotoxicity or graft-versus-host disease were observed. The day 30 and day 100 OR rates were 48.6% for both. The 1-year overall survival and progression-free survival were 68% and 32%, respectively. Patients who achieved OR had higher levels and longer persistence of CAR-NK cells. Receiving CAR-NK cells from a cord blood unit (CBU) with nucleated red blood cells ≤ 8 × 107 and a collection-to-cryopreservation time ≤ 24 h was the most significant predictor for superior outcome. NK cells from these optimal CBUs were highly functional and enriched in effector-related genes. In contrast, NK cells from suboptimal CBUs had upregulation of inflammation, hypoxia and cellular stress programs. Finally, using multiple mouse models, we confirmed the superior antitumor activity of CAR/IL-15 NK cells from optimal CBUs in vivo. These findings uncover new features of CAR-NK cell biology and underscore the importance of donor selection for allogeneic cell therapies. ClinicalTrials.gov identifier: NCT03056339 .
© 2024. The Author(s).
Conflict of interest statement
D.M., Y.L., R.B., H.R., M.D., N.U., S. Acharya, E.L., P.B., M.S., P.L., S. Ang, R.E.C., E.J.S., K.R. and The University of Texas MDACC have an institutional financial conflict of interest with Takeda Pharmaceuticals. D.M., R.B., E.L., S. Ang, E.J.S., K.R. and The University of Texas MDACC have an institutional financial conflict of interest with Affimed. K.R. participates on the Scientific Advisory Board for GemoAb, AvengeBio, Virogin Biotech, GSK, Bayer, Navan Technologies, Caribou Biosciences, Bit Bio and Innate Pharma. K.R. is the scientific founder of Syena. E.J.S. has served on the Scientific Advisory Board for Adaptimmune, Axio, Celaid, FibroBiologics, Navan Technologies, New York Blood Center and Novartis. The remaining authors declare no competing interests.
Figures






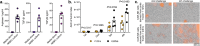



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

