In-vitro susceptibility and ex-vivo evaluation of macrocyclic lactone endectocides sub-lethal concentrations against Plasmodium vivax oocyst development in Anopheles arabiensis
- PMID: 38238768
- PMCID: PMC10797976
- DOI: 10.1186/s12936-024-04845-x
In-vitro susceptibility and ex-vivo evaluation of macrocyclic lactone endectocides sub-lethal concentrations against Plasmodium vivax oocyst development in Anopheles arabiensis
Abstract
Background: Asymptomatic malaria transmission has become a public health concern across malaria-endemic Africa including Ethiopia. Specifically, Plasmodium vivax is more efficient at transmitting earlier in the infection and at lower densities than Plasmodium falciparum. Consequently, a greater proportion of individuals infected with P. vivax can transmit without detectable gametocytaemia. Mass treatment of livestock with macrocyclic lactones (MLs), e.g., ivermectin and doramectin, was suggested as a complementary malaria vector tool because of their insecticidal effects. However, the effects of MLs on P. vivax in Anopheles arabiensis has not yet been fully explored. Hence, comparative in-vitro susceptibility and ex-vivo studies were conducted to evaluate the effects of ivermectin, doramectin and moxidectin sub-lethal concentrations on P. vivax oocyst development in An. arabiensis.
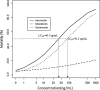
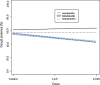
Methods: The 7-day sub-lethal concentrations of 25% (LC25) and 5% (LC5) were determined from in-vitro susceptibility tests on female An. arabiensis in Hemotek® membrane feeding assay. Next, an ex-vivo study was conducted using P. vivax gametocytes infected patient's blood spiked with the LC25 and LC5 of the MLs. At 7-days post-feeding, each mosquito was dissected under a dissection stereo microscope, stained with 0.5% (w/v) mercurochrome solution, and examined for the presence of P. vivax oocysts. Statistical analysis was based on a generalized mixed model with binomially distributed error terms.
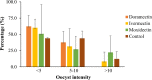
Results: A 7-day lethal concentration of 25% (LC25, in ng/mL) of 7.1 (95% CI: [6.3;8.0]), 20.0 (95%CI:[17.8;22.5]) and 794.3 (95%CI:[716.4;1516.3]) were obtained for ivermectin, doramectin and moxidectin, respectively. Similarly, a lethal concentration of 5% (LC5, in ng/mL) of 0.6 (95% CI: [0.5;0.7]), 1.8 (95% CI:[1.6;2.0]) and 53.7 (95% CI:[ 48.4;102.5]) were obtained respectively for ivermectin, doramectin and moxidectin. The oocyst prevalence in treatment and control groups did not differ significantly (p > 0.05) from each other. Therefore, no direct effect of ML endectocides on P. vivax infection in An. arabiensis mosquitoes was observed at the sub-lethal concentration (LC25 and LC5).
Conclusions: The effects of ivermectin and doramectin on malaria parasite is more likely via indirect effects, particularly by reducing the vectors lifespan and causing mortality before completing the parasite's sporogony cycle or reducing their vector capacity as it affects the locomotor activity of the mosquito.
Keywords: Anopheles arabiensis; Membrane feeding; Oocyst; Plasmodium vivax; Sublethal concentrations.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships and no conflict of interest.
Figures

Similar articles
-
Ivermectin susceptibility, sporontocidal effect, and inhibition of time to re-feed in the Amazonian malaria vector Anopheles darlingi.Malar J. 2017 Nov 21;16(1):474. doi: 10.1186/s12936-017-2125-0. Malar J. 2017. PMID: 29162101 Free PMC article.
-
Similar trends of susceptibility in Anopheles arabiensis and Anopheles pharoensis to Plasmodium vivax infection in Ethiopia.Parasit Vectors. 2016 Oct 18;9(1):552. doi: 10.1186/s13071-016-1839-0. Parasit Vectors. 2016. PMID: 27756355 Free PMC article.
-
Susceptibility of primary, secondary and suspected vectors to Plasmodium vivax and Plasmodium falciparum infection in Ethiopia.Parasit Vectors. 2022 Oct 21;15(1):384. doi: 10.1186/s13071-022-05467-5. Parasit Vectors. 2022. PMID: 36271436 Free PMC article.
-
Systematic review of sporozoite infection rate of Anopheles mosquitoes in Ethiopia, 2001-2021.Parasit Vectors. 2023 Nov 27;16(1):437. doi: 10.1186/s13071-023-06054-y. Parasit Vectors. 2023. PMID: 38008761 Free PMC article. Review.
-
A Roadmap for the Development of Ivermectin as a Complementary Malaria Vector Control Tool.Am J Trop Med Hyg. 2020 Feb;102(2s):3-24. doi: 10.4269/ajtmh.19-0620. Am J Trop Med Hyg. 2020. PMID: 31971144 Free PMC article.
References
-
- WHO. World malaria report 2022 [Internet]. Geneva, World Health Organization, 2022. https://www.who.int/teams/global-malaria-programme/reports/world-malaria...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

