A minimal PBPK model to accelerate preclinical development of drugs against tuberculosis
- PMID: 38239195
- PMCID: PMC10794428
- DOI: 10.3389/fphar.2023.1272091
A minimal PBPK model to accelerate preclinical development of drugs against tuberculosis
Abstract
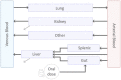
Introduction: Understanding drug exposure at disease target sites is pivotal to profiling new drug candidates in terms of tolerability and efficacy. Such quantification is particularly tedious for anti-tuberculosis (TB) compounds as the heterogeneous pulmonary microenvironment due to the infection may alter lung permeability and affect drug disposition. Murine models have been a longstanding support in TB research so far and are here used as human surrogates to unveil the distribution of several anti-TB compounds at the site-of-action via a novel and centralized PBPK design framework. Methods: As an intermediate approach between data-driven pharmacokinetic (PK) models and whole-body physiologically based (PB) PK models, we propose a parsimonious framework for PK investigation (minimal PBPK approach) that retains key physiological processes involved in TB disease, while reducing computational costs and prior knowledge requirements. By lumping together pulmonary TB-unessential organs, our minimal PBPK model counts 9 equations compared to the 36 of published full models, accelerating the simulation more than 3-folds in Matlab 2022b. Results: The model has been successfully tested and validated against 11 anti-TB compounds-rifampicin, rifapentine, pyrazinamide, ethambutol, isoniazid, moxifloxacin, delamanid, pretomanid, bedaquiline, OPC-167832, GSK2556286 - showing robust predictability power in recapitulating PK dynamics in mice. Structural inspections on the proposed design have ensured global identifiability and listed free fraction in plasma and blood-to-plasma ratio as top sensitive parameters for PK metrics. The platform-oriented implementation allows fast comparison of the compounds in terms of exposure and target attainment. Discrepancies in plasma and lung levels for the latest BPaMZ and HPMZ regimens have been analyzed in terms of their impact on preclinical experiment design and on PK/PD indices. Conclusion: The framework we developed requires limited drug- and species-specific information to reconstruct accurate PK dynamics, delivering a unified viewpoint on anti-TB drug distribution at the site-of-action and a flexible fit-for-purpose tool to accelerate model-informed drug design pipelines and facilitate translation into the clinic.
Keywords: antituberculosis agents; mPBPK; minimal PBPK model; model informed drug development; modeling; simulation; tuberculosis.
Copyright © 2024 Reali, Fochesato, Kaddi, Visintainer, Watson, Levi, Dartois, Azer and Marchetti.
Conflict of interest statement
CK, ML, SW, and KA were employees of Bill and Melinda Gates Medical Research Institute at the time of this work. FR, AF, RV, and LM were contracted by Bill and Melinda Gates Medical Research Institute while this research was conducted. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- Allué-Guardia A., Garcia-Vilanova A., Olmo-Fontánez A. M., Peters J., Maselli D. J., Wang Y., et al. (2022). Host- and age-dependent transcriptional changes in Mycobacterium tuberculosis cell envelope biosynthesis genes after exposure to human alveolar lining fluid. Int. J. Mol. Sci. 23 (2), 983. Available at:. 10.3390/ijms23020983 - DOI - PMC - PubMed
-
- Auger A., Hansen N. (2012). Tutorial CMA-ES: evolution strategies and covariance matrix adaptation. GECCO (Companion), 1–80. Available at:. 10.1145/2001858.2002123 - DOI
-
- Bloomingdale P., Bakshi S., Maass C., van Maanen E., Pichardo-Almarza C., Yadav D. B., et al. (2021). Minimal brain PBPK model to support the preclinical and clinical development of antibody therapeutics for CNS diseases. J. Pharmacokinet. pharmacodynamics 48 (6), 861–871. Available at:. 10.1007/s10928-021-09776-7 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

