Development of the sleep-wake switch in rats during the P2-P21 early infancy period
- PMID: 38239232
- PMCID: PMC10794532
- DOI: 10.3389/fnetp.2023.1340722
Development of the sleep-wake switch in rats during the P2-P21 early infancy period
Abstract
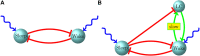
In early infancy, rats randomly alternate between the sleeping and waking states-from postnatal day 2-10 (P2-P10), sleep and wake bouts are both exponentially distributed with increasing means, while from P10-P21 sleep and wake bout means continue to increase, though there is a striking qualitative shift in the distribution of wake bouts from exponential to power law. The behavioral states of sleep and wakefulness correspond to the activity of sleep-active and wake-active neuronal brainstem populations, with reciprocal inhibition between the two ensuring that only one population is active at a time. The locus coeruleus (LC) forms a third component of this circuit that rises in prominence during the P10-P21 period, as experimental evidence shows that an as-of-yet undeciphered interaction of the LC with sleep-active and wake-active populations is responsible for the transformation of the wake bout distribution from exponential to power law. Interestingly, the LC undergoes remarkable physiological changes during the P10-P21 period-gap junctions within the LC are pruned and network-wide oscillatory synchrony declines and vanishes. In this work, we discuss a series of models of sleep-active, wake-active, and the LC populations, and we use these models to postulate the nature of the interaction between these three populations and how these interactions explain empirical observations of sleep and wake bout dynamics. We hypothesize a circuit in which there is reciprocal excitation between the LC and wake-active population with inhibition from the sleep-active population to the LC that suppresses the LC during sleep bouts. During the P2-P10 period, we argue that a noise-based switching mechanism between the sleep-active and wake-active populations provides a simple and natural way to account for exponential bout distributions, and that the locked oscillatory state of the LC prevents it from impacting bout distributions. From P10-P21, we use our models to postulate that, as the LC gradually shifts from a state of synchronized oscillations to a state of continuous firing, reciprocal excitation between the LC and the wake-active population is able to gradually transform the wake bout distribution from exponential to power law.
Keywords: infant development; locus coeruleus; power law; reciprocal inhibition; sleep wake.
Copyright © 2024 Patel and Joshi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous