Analysis of α-syn and parkin interaction in mediating neuronal death in Drosophila model of Parkinson's disease
- PMID: 38239290
- PMCID: PMC10794313
- DOI: 10.3389/fncel.2023.1295805
Analysis of α-syn and parkin interaction in mediating neuronal death in Drosophila model of Parkinson's disease
Abstract
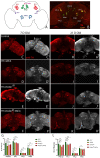
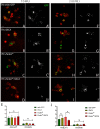
One of the hallmarks of Parkinson's Disease (PD) is aggregation of incorrectly folded α-synuclein (SNCA) protein resulting in selective death of dopaminergic neurons. Another form of PD is characterized by the loss-of-function of an E3-ubiquitin ligase, parkin. Mutations in SNCA and parkin result in impaired mitochondrial morphology, causing loss of dopaminergic neurons. Despite extensive research on the individual effects of SNCA and parkin, their interactions in dopaminergic neurons remain understudied. Here we employ Drosophila model to study the effect of collective overexpression of SNCA along with the downregulation of parkin in the dopaminergic neurons of the posterior brain. We found that overexpression of SNCA along with downregulation of parkin causes a reduction in the number of dopaminergic neuronal clusters in the posterior region of the adult brain, which is manifested as progressive locomotor dysfunction. Overexpression of SNCA and downregulation of parkin collectively results in altered mitochondrial morphology in a cluster-specific manner, only in a subset of dopaminergic neurons of the brain. Further, we found that SNCA overexpression causes transcriptional downregulation of parkin. However, this downregulation is not further enhanced upon collective SNCA overexpression and parkin downregulation. This suggests that the interactions of SNCA and parkin may not be additive. Our study thus provides insights into a potential link between α-synuclein and parkin interactions. These interactions result in altered mitochondrial morphology in a cluster-specific manner for dopaminergic neurons over a time, thus unraveling the molecular interactions involved in the etiology of Parkinson's Disease.
Keywords: Drosophila melanogaster; Parkinson's disease; dopaminergic neurons; mitochondrial morphology; parkin; tyrosine hydroxylase; α-synuclein.
Copyright © 2024 Narwal, Singh and Tare.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

