High-throughput CRISPR technology: a novel horizon for solid organ transplantation
- PMID: 38239344
- PMCID: PMC10794540
- DOI: 10.3389/fimmu.2023.1295523
High-throughput CRISPR technology: a novel horizon for solid organ transplantation
Abstract
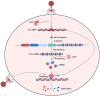
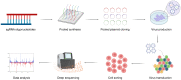
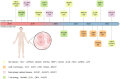
Organ transplantation is the gold standard therapy for end-stage organ failure. However, the shortage of available grafts and long-term graft dysfunction remain the primary barriers to organ transplantation. Exploring approaches to solve these issues is urgent, and CRISPR/Cas9-based transcriptome editing provides one potential solution. Furthermore, combining CRISPR/Cas9-based gene editing with an ex vivo organ perfusion system would enable pre-implantation transcriptome editing of grafts. How to determine effective intervention targets becomes a new problem. Fortunately, the advent of high-throughput CRISPR screening has dramatically accelerated the effective targets. This review summarizes the current advancements, utilization, and workflow of CRISPR screening in various immune and non-immune cells. It also discusses the ongoing applications of CRISPR/Cas-based gene editing in transplantation and the prospective applications of CRISPR screening in solid organ transplantation.
Keywords: CRISPR screening; CRISPR/Cas9; high-throughput; solid organ transplantation; transcriptome editing.
Copyright © 2024 Li, Chen, Ye, Yu, Zhang, Li, Niu, Ran, Wang, Luo, Zhao, Hao, Zong, Xia, Xia and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Gene Therapy with CRISPR/Cas9 Coming to Age for HIV Cure.AIDS Rev. 2017 Oct-Dec;19(3):167-172. AIDS Rev. 2017. PMID: 29019352
-
Genome Editing with CRISPR-Cas9: A Budding Biological Contrivance for Colorectal Carcinoma Research and its Perspective in Molecular Medicine.Curr Mol Med. 2021;21(6):462-475. doi: 10.2174/1566524020666201119143943. Curr Mol Med. 2021. PMID: 33213345
-
Recent advances in the delivery and applications of nonviral CRISPR/Cas9 gene editing.Drug Deliv Transl Res. 2023 May;13(5):1500-1519. doi: 10.1007/s13346-023-01320-z. Epub 2023 Mar 29. Drug Deliv Transl Res. 2023. PMID: 36988873 Free PMC article. Review.
-
Utilization of CRISPR/Cas9 gene editing in cellular therapies for lymphoid malignancies.Immunol Lett. 2020 Oct;226:71-82. doi: 10.1016/j.imlet.2020.07.003. Epub 2020 Jul 17. Immunol Lett. 2020. PMID: 32687855 Review.
-
Development of a CRISPR/Cas9 System for Methylococcus capsulatus In Vivo Gene Editing.Appl Environ Microbiol. 2019 May 16;85(11):e00340-19. doi: 10.1128/AEM.00340-19. Print 2019 Jun 1. Appl Environ Microbiol. 2019. PMID: 30926729 Free PMC article.
Cited by
-
Comparative analysis and process optimization for manufacturing CAR-T using the PiggyBac system derived from cryopreserved versus fresh PBMCs.Sci Rep. 2025 Feb 11;15(1):5023. doi: 10.1038/s41598-025-89686-7. Sci Rep. 2025. PMID: 39934258 Free PMC article.
-
Advances in CRISPR-Cas9 in lineage tracing of model animals.Animal Model Exp Med. 2025 Jun;8(6):1004-1022. doi: 10.1002/ame2.70033. Epub 2025 Jun 10. Animal Model Exp Med. 2025. PMID: 40491322 Free PMC article. Review.
-
Research Progress on the Preparation and Application of Decellularized Tendons.Curr Issues Mol Biol. 2025 Apr 6;47(4):251. doi: 10.3390/cimb47040251. Curr Issues Mol Biol. 2025. PMID: 40699650 Free PMC article. Review.
-
Exploring Monkeypox: prospects for therapeutics through computational-aided drug discovery.Mol Divers. 2024 Oct;28(5):3497-3521. doi: 10.1007/s11030-023-10767-8. Epub 2023 Dec 11. Mol Divers. 2024. PMID: 38079063 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

