Update on novel synthetic approaches towards the construction of carbazole nuclei: a review
- PMID: 38239436
- PMCID: PMC10794906
- DOI: 10.1039/d3ra07270c
Update on novel synthetic approaches towards the construction of carbazole nuclei: a review
Abstract
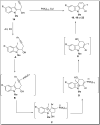
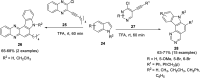
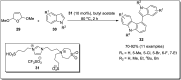
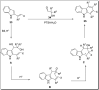
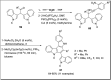
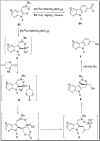
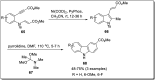
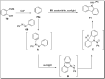
The carbazole scaffold is a significant entity in organic compounds due to its variety of biological and synthetic applications. Traditionally, carbazole skeletons have been synthesized either via the Grabe-Ullman method, Clemo-Perkin method or Tauber method. With the passage of time, these methods have been modified and explored to accomplish the synthesis of target compounds. These methods include hydroarylations, C-H activations, annulations and cyclization reactions mediated by a variety of catalysts to construct carbazole-based compounds. This brief review article intends to provide recent updates on important methodological developments reported for the synthesis of carbazole nuclei covering 2019-2023.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Recent synthetic strategies for the construction of functionalized carbazoles and their heterocyclic motifs enabled by Lewis acids.RSC Adv. 2023 Nov 6;13(46):32596-32626. doi: 10.1039/d3ra06396h. eCollection 2023 Oct 31. RSC Adv. 2023. PMID: 37936643 Free PMC article. Review.
-
[Development of New Synthetic Methods for Carbazole Compounds Aimed at Drug Discovery and Exploratory Research on Pharmaceutical Materials].Yakugaku Zasshi. 2021;141(12):1281-1288. doi: 10.1248/yakushi.21-00141. Yakugaku Zasshi. 2021. PMID: 34853199 Review. Japanese.
-
Total synthesis of indoline alkaloids: A cyclopropanation strategy.Acc Chem Res. 2011 Jun 21;44(6):447-57. doi: 10.1021/ar200004w. Epub 2011 Apr 14. Acc Chem Res. 2011. PMID: 21491859
-
Synthetic Approaches to Tetracyclic Indolines as Versatile Building Blocks of Diverse Indole Alkaloids.Chemistry. 2018 Sep 25;24(54):14302-14315. doi: 10.1002/chem.201800775. Epub 2018 Jul 6. Chemistry. 2018. PMID: 29624769 Review.
-
Insights from Binding on Quadruplex Selective Carbazole Ligands.Chemistry. 2021 Sep 6;27(50):12726-12736. doi: 10.1002/chem.202101866. Epub 2021 Jul 19. Chemistry. 2021. PMID: 34138492 Free PMC article. Review.
References
-
- Al-Mulla A. A Review: Biological Importance of Heterocyclic Compounds. Pharma Chem. 2017;9:141–147.
-
- Shahzadi I. Zahoor A. F. Rasul A. Rasool N. Raza Z. Faisal S. Faisal S. Parveen B. Kamal S. Rehman M. Z. Zahid F. M. Synthesis, Anticancer, and Computational Studies of 1, 3, 4-Oxadiazole-Purine Derivatives. J. Heterocycl. Chem. 2020;57:2782–2794.
-
- Cinar M. E. Ozturk T. Thienothiophenes, Dithienothiophenes, and Thienoacenes: Syntheses, Oligomers, Polymers, and Properties. Chem. Rev. 2015;115:3036–3140. - PubMed
-
- Goncalves M. S. T. Fluorescent Labeling of Biomolecules with Organic Probes. Chem. Rev. 2009;109(1):190–212. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

