Plasma metabolites distinguish dementia with Lewy bodies from Alzheimer's disease: a cross-sectional metabolomic analysis
- PMID: 38239488
- PMCID: PMC10794326
- DOI: 10.3389/fnagi.2023.1326780
Plasma metabolites distinguish dementia with Lewy bodies from Alzheimer's disease: a cross-sectional metabolomic analysis
Abstract
Background: In multifactorial diseases, alterations in the concentration of metabolites can identify novel pathological mechanisms at the intersection between genetic and environmental influences. This study aimed to profile the plasma metabolome of patients with dementia with Lewy bodies (DLB) and Alzheimer's disease (AD), two neurodegenerative disorders for which our understanding of the pathophysiology is incomplete. In the clinical setting, DLB is often mistaken for AD, highlighting a need for accurate diagnostic biomarkers. We therefore also aimed to determine the overlapping and differentiating metabolite patterns associated with each and establish whether identification of these patterns could be leveraged as biomarkers to support clinical diagnosis.
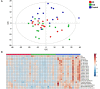
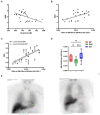
Methods: A panel of 630 metabolites (Biocrates MxP Quant 500) and a further 232 metabolism indicators (biologically informative sums and ratios calculated from measured metabolites, each indicative for a specific pathway or synthesis; MetaboINDICATOR) were analyzed in plasma from patients with probable DLB (n = 15; age 77.6 ± 8.2 years), probable AD (n = 15; 76.1 ± 6.4 years), and age-matched cognitively healthy controls (HC; n = 15; 75.2 ± 6.9 years). Metabolites were quantified using a reversed-phase ultra-performance liquid chromatography column and triple-quadrupole mass spectrometer in multiple reaction monitoring (MRM) mode, or by using flow injection analysis in MRM mode. Data underwent multivariate (PCA analysis), univariate and receiving operator characteristic (ROC) analysis. Metabolite data were also correlated (Spearman r) with the collected clinical neuroimaging and protein biomarker data.
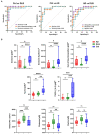
Results: The PCA plot separated DLB, AD and HC groups (R2 = 0.518, Q2 = 0.348). Significant alterations in 17 detected metabolite parameters were identified (q ≤ 0.05), including neurotransmitters, amino acids and glycerophospholipids. Glutamine (Glu; q = 0.045) concentrations and indicators of sphingomyelin hydroxylation (q = 0.039) distinguished AD and DLB, and these significantly correlated with semi-quantitative measurement of cardiac sympathetic denervation. The most promising biomarker differentiating AD from DLB was Glu:lysophosphatidylcholine (lysoPC a 24:0) ratio (AUC = 0.92; 95%CI 0.809-0.996; sensitivity = 0.90; specificity = 0.90).
Discussion: Several plasma metabolomic aberrations are shared by both DLB and AD, but a rise in plasma glutamine was specific to DLB. When measured against plasma lysoPC a C24:0, glutamine could differentiate DLB from AD, and the reproducibility of this biomarker should be investigated in larger cohorts.
Keywords: Alzheimer’s disease; Lewy body dementia; biomarkers; dementia; metabolomic analyses.
Copyright © 2024 Pan, Donaghy, Roberts, Chouliaras, O’Brien, Thomas, Heslegrave, Zetterberg, McGuinness, Passmore, Green and Kane.
Conflict of interest statement
The Newcastle upon Tyne Hospitals Nuclear Medicine department receives funding from GE Healthcare for educational presentations delivered by GR. Outside of this work JO’B has acted as a consultant for TauRx, Novo Nordisk, Biogen, Roche, Lilly and GE Healthcare and received grant or academic support from Avid/Lilly, Merck and Alliance Medical. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Alimov I. I., Nazhmidinova M. N., Ganiev K. G. (1990). Changes in lipid metabolism in patients with Parkinson disease after combined treatment with L-deprenil (iumex) and antiparkinson drugs. Zh. Nevropatol. Psikhiatr. Im. S. S. Korsakova 90, 35–38. - PubMed
-
- Aquilani R., Costa A., Maestri R., Cotta Ramusino M., Pierobon A., Dossena M., et al. (2020). Mini nutritional assessment May identify a dual pattern of perturbed plasma amino acids in patients with Alzheimer's disease: a window to metabolic and physical rehabilitation? Nutrients 12:1845. doi: 10.3390/nu12061845, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

