Spatial profiling of the placental chorioamniotic membranes reveals upregulation of immune checkpoint proteins during Group B Streptococcus infection in a nonhuman primate model
- PMID: 38239507
- PMCID: PMC10794649
- DOI: 10.3389/fcimb.2023.1299644
Spatial profiling of the placental chorioamniotic membranes reveals upregulation of immune checkpoint proteins during Group B Streptococcus infection in a nonhuman primate model
Abstract
Background: Preterm birth is a leading cause of neonatal mortality, which is often complicated by intrauterine infection and inflammation. We have established a nonhuman primate model of Group B Streptococcus (GBS, Streptococcus agalactiae) infection-associated preterm birth. Immune checkpoints are modulators of the immune response by activating or suppressing leukocyte function and are understudied in preterm birth. The objective of this study was to spatially profile changes in immune protein expression at the maternal-fetal interface during a GBS infection with a focus on immune checkpoints.
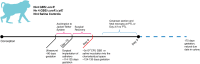
Methods: Twelve nonhuman primates (pigtail macaques, Macaca nemestrina) received a choriodecidual inoculation of either: 1) 1-5 X 108 colony forming units (CFU) of hyperhemolytic/hypervirulent GBS (GBSΔcovR, N=4); 2) an isogenic/nonpigmented strain (GBS ΔcovRΔcylE, N=4); or, 3) saline (N=4). A Cesarean section was performed at preterm labor or 3 days after GBS infection or 7 days after saline inoculation. Nanostring GeoMx® Digital Spatial Profiling technology was used to segment protein expression within the amnion, chorion, and maternal decidua at the inoculation site using an immuno-oncology panel targeting 56 immunoproteins enriched in stimulatory and inhibitory immune checkpoint proteins or their protein ligands. Statistical analysis included R studio, Kruskal-Wallis, Pearson and Spearman tests.
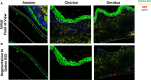
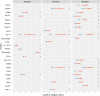
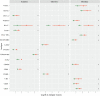
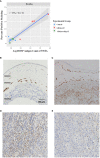
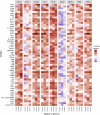
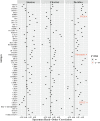
Results: Both inhibitory and stimulatory immune checkpoint proteins were significantly upregulated within the chorioamniotic membranes and decidua (VISTA, LAG3, PD-1, CD40, GITR), as well as their ligands (PD-L1, PD-L2, CD40L; all p<0.05). Immunostaining for VISTA revealed positive (VISTA+) cells, predominantly in the chorion and decidua. There were strong correlations between VISTA and amniotic fluid concentrations of IL-1β, IL-6, IL-8, and TNF-α (all p<0.05), as well as maternal placental histopathology scores (p<0.05).
Conclusion: Differential regulation of multiple immune checkpoint proteins in the decidua at the site of a GBS infection indicates a major perturbation in immunologic homeostasis that could benefit the host by restricting immune-driven pathologies or the pathogen by limiting immune surveillance. Protein expression of VISTA, an inhibitory immune checkpoint, was upregulated in the chorion and decidua after GBS infection. Investigating the impact of innate immune cell expression of inhibitory immune checkpoints may reveal new insights into placental host-pathogen interactions at the maternal-fetal interface.
Keywords: Group B Streptococcus; amnion; chorion; decidua; immune checkpoint; placenta; pregnancy.
Copyright © 2024 Manuel, Coleman, Orvis, Munson, Li, Kapur, Li, Li, Armistead, Rajagopal and Adams Waldorf.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Amniotic fluid interleukin 6 and interleukin 8 are superior predictors of fetal lung injury compared with maternal or fetal plasma cytokines or placental histopathology in a nonhuman primate model.Am J Obstet Gynecol. 2021 Jul;225(1):89.e1-89.e16. doi: 10.1016/j.ajog.2020.12.1214. Epub 2021 Jan 4. Am J Obstet Gynecol. 2021. PMID: 33412130 Free PMC article.
-
A Broad Spectrum Chemokine Inhibitor Prevents Preterm Labor but Not Microbial Invasion of the Amniotic Cavity or Neonatal Morbidity in a Non-human Primate Model.Front Immunol. 2020 Apr 30;11:770. doi: 10.3389/fimmu.2020.00770. eCollection 2020. Front Immunol. 2020. PMID: 32425945 Free PMC article.
-
Choriodecidual group B streptococcal inoculation induces fetal lung injury without intra-amniotic infection and preterm labor in Macaca nemestrina.PLoS One. 2011;6(12):e28972. doi: 10.1371/journal.pone.0028972. Epub 2011 Dec 21. PLoS One. 2011. PMID: 22216148 Free PMC article.
-
Mechanisms of group B Streptococcus-mediated preterm birth: lessons learnt from animal models.Reprod Fertil. 2022 Jun 7;3(3):R109-R120. doi: 10.1530/RAF-21-0105. eCollection 2022 Jul 1. Reprod Fertil. 2022. PMID: 35794927 Free PMC article. Review.
-
Preventing neonatal group B streptococcal infection. Intrapartum antibiotic prophylaxis in some high-risk situations.Prescrire Int. 2011 Mar;20(114):72-7. Prescrire Int. 2011. PMID: 21648230 Review.
Cited by
-
Immune checkpoint for pregnancy.Semin Immunopathol. 2025 May 2;47(1):26. doi: 10.1007/s00281-025-01051-y. Semin Immunopathol. 2025. PMID: 40314833 Review.
References
-
- Adams Waldorf K. M., Gravett M. G., McAdams R. M., Paolella L. J., Gough G. M., Carl D. J., et al. . (2011. a). Choriodecidual group B streptococcal inoculation induces fetal lung injury without intra-amniotic infection and preterm labor in Macaca nemestrina. PloS One 6 (12), e28972. doi: 10.1371/journal.pone.0028972 - DOI - PMC - PubMed
-
- Bianchi-Jassir F., Seale A. C., Kohli-Lynch M., Lawn J. E., Baker C. J., Bartlett L., et al. . (2017). Preterm birth associated with group B streptococcus maternal colonization worldwide: systematic review and meta-analyses. Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. America 65 (suppl_2), S133–SS42. doi: 10.1093/cid/cix661 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

