Signalling mechanisms and agricultural applications of (Z)-3-hexenyl butyrate-mediated stomatal closure
- PMID: 38239809
- PMCID: PMC10794947
- DOI: 10.1093/hr/uhad248
Signalling mechanisms and agricultural applications of (Z)-3-hexenyl butyrate-mediated stomatal closure
Abstract
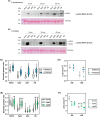
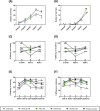
Biotic and abiotic stresses can severely limit crop productivity. In response to drought, plants close stomata to prevent water loss. Furthermore, stomata are the main entry point for several pathogens. Therefore, the development of natural products to control stomata closure can be considered a sustainable strategy to cope with stresses in agriculture. Plants respond to different stresses by releasing volatile organic compounds. Green leaf volatiles, which are commonly produced across different plant species after tissue damage, comprise an important group within volatile organic compounds. Among them, (Z)-3-hexenyl butyrate (HB) was described as a natural inducer of stomatal closure, playing an important role in stomatal immunity, although its mechanism of action is still unknown. Through different genetic, pharmacological, and biochemical approaches, we here uncover that HB perception initiates various defence signalling events, such as activation of Ca2+ permeable channels, mitogen-activated protein kinases, and production of Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-mediated reactive oxygen species. Furthermore, HB-mediated stomata closure was found to be independent of abscisic acid biosynthesis and signalling. Additionally, exogenous treatments with HB alleviate water stress and improve fruit productivity in tomato plants. The efficacy of HB was also tested under open field conditions, leading to enhanced resistance against Phytophthora spp. and Pseudomonas syringae infection in potato and tomato plants, respectively. Taken together, our results provide insights into the HB signalling transduction pathway, confirming its role in stomatal closure and plant immune system activation, and propose HB as a new phytoprotectant for the sustainable control of biotic and abiotic stresses in agriculture.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nanjing Agricultural University.
Conflict of interest statement
None declared.
Figures




References
-
- Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–9 - PubMed
-
- Boutrot F, Zipfel C. Function, discovery, and exploitation of plant pattern recognition receptors for broad-spectrum disease resistance. Annu Rev Phytopathol. 2017;55:257–86 - PubMed
-
- Zhang J, Zhou JM. Plant immunity triggered by microbial molecular signatures. Mol Plant. 2010;3:783–93 - PubMed
-
- Cui H, Tsuda K, Parker JE. Effector-triggered immunity: from pathogen perception to robust defense. Annu Rev Plant Biol. 2015;66:487–511 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

