A Natural Small Molecule Mitigates Kidney Fibrosis by Targeting Cdc42-mediated GSK-3β/β-catenin Signaling
- PMID: 38240457
- PMCID: PMC10987128
- DOI: 10.1002/advs.202307850
A Natural Small Molecule Mitigates Kidney Fibrosis by Targeting Cdc42-mediated GSK-3β/β-catenin Signaling
Abstract
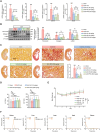
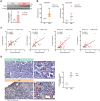
Kidney fibrosis is a common fate of chronic kidney diseases (CKDs), eventually leading to renal dysfunction. Yet, no effective treatment for this pathological process has been achieved. During the bioassay-guided chemical investigation of the medicinal plant Wikstroemia chamaedaphne, a daphne diterpenoid, daphnepedunin A (DA), is characterized as a promising anti-renal fibrotic lead. DA shows significant anti-kidney fibrosis effects in cultured renal fibroblasts and unilateral ureteral obstructed mice, being more potent than the clinical trial drug pirfenidone. Leveraging the thermal proteome profiling strategy, cell division cycle 42 (Cdc42) is identified as the direct target of DA. Mechanistically, DA targets to reduce Cdc42 activity and down-regulates its downstream phospho-protein kinase Cζ(p-PKCζ)/phospho-glycogen synthase kinase-3β (p-GSK-3β), thereby promoting β-catenin Ser33/37/Thr41 phosphorylation and ubiquitin-dependent proteolysis to block classical pro-fibrotic β-catenin signaling. These findings suggest that Cdc42 is a promising therapeutic target for kidney fibrosis, and highlight DA as a potent Cdc42 inhibitor for combating CKDs.
Keywords: Cdc42; GSK‐3β/β‐catenin; kidney fibrosis; natural small molecules.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Collaboration G. B. D. C. K. D., Lancet 2020, 395, 709. - PubMed
-
- Rockey D. C., Bell P. D., Hill J. A., N. Engl. J. Med. 2015, 372, 1138. - PubMed
-
- a) Kuehn B. M., JAMA, J. Am. Med. Assoc. 2022, 327, 1540;
- b) Ruiz‐Ortega M., Rayego‐Mateos S., Lamas S., Ortiz A., Rodrigues‐Diez R. R., Nat. Rev. Nephrol. 2020, 16, 269. - PubMed
-
- Ruiz‐Ortega M., Lamas S., Ortiz A., Am. J. Kidney Dis. 2022, 80, 251. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
