A Fast, Convenient, Polarizable Electrostatic Model for Molecular Dynamics
- PMID: 38240687
- PMCID: PMC10867846
- DOI: 10.1021/acs.jctc.3c01171
A Fast, Convenient, Polarizable Electrostatic Model for Molecular Dynamics
Abstract
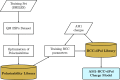
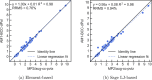
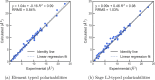
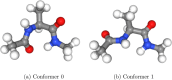
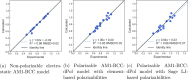
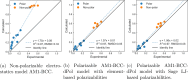
We present an efficient polarizable electrostatic model, utilizing typed, atom-centered polarizabilities and the fast direct approximation, designed for efficient use in molecular dynamics (MD) simulations. The model provides two convenient approaches for assigning partial charges in the context of atomic polarizabilities. One is a generalization of RESP, called RESP-dPol, and the other, AM1-BCC-dPol, is an adaptation of the widely used AM1-BCC method. Both are designed to accurately replicate gas-phase quantum mechanical electrostatic potentials. Benchmarks of this polarizable electrostatic model against gas-phase dipole moments, molecular polarizabilities, bulk liquid densities, and static dielectric constants of organic liquids show good agreement with the reference values. Of note, the model yields markedly more accurate dielectric constants of organic liquids, relative to a matched nonpolarizable force field. MD simulations with this method, which is currently parametrized for molecules containing elements C, N, O, and H, run only about 3.6-fold slower than fixed charge force fields, while simulations with the self-consistent mutual polarization average 4.5-fold slower. Our results suggest that RESP-dPol and AM1-BCC-dPol afford improved accuracy relative to fixed charge force fields and are good starting points for developing general, affordable, and transferable polarizable force fields. The software implementing these approaches has been designed to utilize the force field fitting frameworks developed and maintained by the Open Force Field Initiative, setting the stage for further exploration of this approach to polarizable force field development.
Conflict of interest statement
The authors declare the following competing financial interest(s): M.K.G. has an equity interest in and is a cofounder and scientific advisor of VeraChem LLC. He is also on the Scientific Advisory Boards of Denovicon and In Cerebro. D.L.M. serves on the scientific advisory boards of OpenEye Scientific Software, Cadence Molecular Sciences, and Anagenex, and is an Open Science Fellow with Psivant Sciences.
Figures
References
-
- Brooks B. R.; Bruccoleri R. E.; Olafson B. D.; States D. J.; Swaminathan S.; Karplus M. CHARMM: A program for macromolecular energy, minimization, and dynamics calculations. J. Comput. Chem. 1983, 4, 187–217. 10.1002/jcc.540040211. - DOI
-
- Boothroyd S.; Behara P. K.; Madin O. C.; Hahn D. F.; Jang H.; Gapsys V.; Wagner J. R.; Horton J. T.; Dotson D. L.; Thompson M. W.; Maat J.; Gokey T.; Wang L.-P.; Cole D. J.; Gilson M. K.; Chodera J. D.; Bayly C. I.; Shirts M. R.; Mobley D. L. Development and Benchmarking of Open Force Field 2.0.0: The Sage Small Molecule Force Field. J. Chem. Theory Comput. 2023, 19, 3251–3275. 10.1021/acs.jctc.3c00039. - DOI - PMC - PubMed
-
- Bayly C. I.; Cieplak P.; Cornell W.; Kollman P. A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J. Phys. Chem. 1993, 97, 10269–10280. 10.1021/j100142a004. - DOI
