Field assessment of the operating procedures of a semi-quantitative G6PD Biosensor to improve repeatability of routine testing
- PMID: 38241389
- PMCID: PMC10798449
- DOI: 10.1371/journal.pone.0296708
Field assessment of the operating procedures of a semi-quantitative G6PD Biosensor to improve repeatability of routine testing
Erratum in
-
Correction: Field assessment of the operating procedures of a semi-quantitative G6PD Biosensor to improve repeatability of routine testing.PLoS One. 2024 Feb 29;19(2):e0300016. doi: 10.1371/journal.pone.0300016. eCollection 2024. PLoS One. 2024. PMID: 38421975 Free PMC article.
Abstract
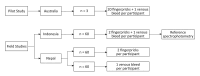
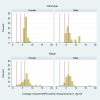
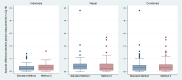
In remote communities, diagnosis of G6PD deficiency is challenging. We assessed the impact of modified test procedures and delayed testing for the point-of-care diagnostic STANDARD G6PD (SDBiosensor, RoK), and evaluated recommended cut-offs. We tested capillary blood from fingerpricks (Standard Method) and a microtainer (BD, USA; Method 1), venous blood from a vacutainer (BD, USA; Method 2), varied sample application methods (Methods 3), and used micropipettes rather than the test's single-use pipette (Method 4). Repeatability was assessed by comparing median differences between paired measurements. All methods were tested 20 times under laboratory conditions on three volunteers. The Standard Method and the method with best repeatability were tested in Indonesia and Nepal. In Indonesia 60 participants were tested in duplicate by both methods, in Nepal 120 participants were tested in duplicate by either method. The adjusted male median (AMM) of the Biosensor Standard Method readings was defined as 100% activity. In Indonesia, the difference between paired readings of the Standard and modified methods was compared to assess the impact of delayed testing. In the pilot study repeatability didn't differ significantly (p = 0.381); Method 3 showed lowest variability. One Nepalese participant had <30% activity, one Indonesian and 10 Nepalese participants had intermediate activity (≥30% to <70% activity). Repeatability didn't differ significantly in Indonesia (Standard: 0.2U/gHb [IQR: 0.1-0.4]; Method 3: 0.3U/gHb [IQR: 0.1-0.5]; p = 0.425) or Nepal (Standard: 0.4U/gHb [IQR: 0.2-0.6]; Method 3: 0.3U/gHb [IQR: 0.1-0.6]; p = 0.330). Median G6PD measurements by Method 3 were 0.4U/gHb (IQR: -0.2 to 0.7, p = 0.005) higher after a 5-hour delay compared to the Standard Method. The definition of 100% activity by the Standard Method matched the manufacturer-recommended cut-off for 70% activity. We couldn't improve repeatability. Delays of up to 5 hours didn't result in a clinically relevant difference in measured G6PD activity. The manufacturer's recommended cut-off for intermediate deficiency is conservative.
Copyright: © 2024 Sadhewa et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Performance of quantitative point-of-care tests to measure G6PD activity: An individual participant data meta-analysis.PLoS Negl Trop Dis. 2025 Mar 25;19(3):e0012864. doi: 10.1371/journal.pntd.0012864. eCollection 2025 Mar. PLoS Negl Trop Dis. 2025. PMID: 40132008 Free PMC article.
-
Repeatability and reproducibility of a handheld quantitative G6PD diagnostic.PLoS Negl Trop Dis. 2022 Feb 17;16(2):e0010174. doi: 10.1371/journal.pntd.0010174. eCollection 2022 Feb. PLoS Negl Trop Dis. 2022. PMID: 35176015 Free PMC article.
-
Evaluation of a point-of-care diagnostic to identify glucose-6-phosphate dehydrogenase deficiency in Brazil.PLoS Negl Trop Dis. 2021 Aug 12;15(8):e0009649. doi: 10.1371/journal.pntd.0009649. eCollection 2021 Aug. PLoS Negl Trop Dis. 2021. PMID: 34383774 Free PMC article.
-
Field evaluation of a novel semi-quantitative point-of-care diagnostic for G6PD deficiency in Indonesia.PLoS One. 2024 Apr 30;19(4):e0301506. doi: 10.1371/journal.pone.0301506. eCollection 2024. PLoS One. 2024. PMID: 38687748 Free PMC article.
-
Quantification of glucose-6-phosphate dehydrogenase activity by spectrophotometry: A systematic review and meta-analysis.PLoS Med. 2020 May 14;17(5):e1003084. doi: 10.1371/journal.pmed.1003084. eCollection 2020 May. PLoS Med. 2020. PMID: 32407380 Free PMC article.
Cited by
-
Correction: Field assessment of the operating procedures of a semi-quantitative G6PD Biosensor to improve repeatability of routine testing.PLoS One. 2024 Feb 29;19(2):e0300016. doi: 10.1371/journal.pone.0300016. eCollection 2024. PLoS One. 2024. PMID: 38421975 Free PMC article.
-
Performance of quantitative point-of-care tests to measure G6PD activity: An individual participant data meta-analysis.PLoS Negl Trop Dis. 2025 Mar 25;19(3):e0012864. doi: 10.1371/journal.pntd.0012864. eCollection 2025 Mar. PLoS Negl Trop Dis. 2025. PMID: 40132008 Free PMC article.
References
-
- World Health Organization. World Malaria Report 2022. Geneva: World Health Organization, 2022. 8 December 2022. Report No.: 978 92 4 006489 8.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

