SAGA1 and SAGA2 promote starch formation around proto-pyrenoids in Arabidopsis chloroplasts
- PMID: 38241434
- PMCID: PMC10823261
- DOI: 10.1073/pnas.2311013121
SAGA1 and SAGA2 promote starch formation around proto-pyrenoids in Arabidopsis chloroplasts
Abstract
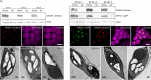
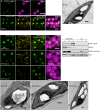
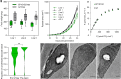
The pyrenoid is a chloroplastic microcompartment in which most algae and some terrestrial plants condense the primary carboxylase, Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase) as part of a CO2-concentrating mechanism that improves the efficiency of CO2 capture. Engineering a pyrenoid-based CO2-concentrating mechanism (pCCM) into C3 crop plants is a promising strategy to enhance yield capacities and resilience to the changing climate. Many pyrenoids are characterized by a sheath of starch plates that is proposed to act as a barrier to limit CO2 diffusion. Recently, we have reconstituted a phase-separated "proto-pyrenoid" Rubisco matrix in the model C3 plant Arabidopsis thaliana using proteins from the alga with the most well-studied pyrenoid, Chlamydomonas reinhardtii [N. Atkinson, Y. Mao, K. X. Chan, A. J. McCormick, Nat. Commun. 11, 6303 (2020)]. Here, we describe the impact of introducing the Chlamydomonas proteins StArch Granules Abnormal 1 (SAGA1) and SAGA2, which are associated with the regulation of pyrenoid starch biogenesis and morphology. We show that SAGA1 localizes to the proto-pyrenoid in engineered Arabidopsis plants, which results in the formation of atypical spherical starch granules enclosed within the proto-pyrenoid condensate and adjacent plate-like granules that partially cover the condensate, but without modifying the total amount of chloroplastic starch accrued. Additional expression of SAGA2 further increases the proportion of starch synthesized as adjacent plate-like granules that fully encircle the proto-pyrenoid. Our findings pave the way to assembling a diffusion barrier as part of a functional pCCM in vascular plants, while also advancing our understanding of the roles of SAGA1 and SAGA2 in starch sheath formation and broadening the avenues for engineering starch morphology.
Keywords: Arabidopsis; CO2-concentrating mechanism; Chlamydomonas; EPYC1; Rubisco.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Tcherkez G., The mechanism of Rubisco-catalysed oxygenation. Plant Cell Environ. 39, 983–997 (2016). - PubMed
-
- Walker B. J., Vanloocke A., Bernacchi C. J., Ort D. R., The Costs of photorespiration to food production now and in the future. Annu. Rev. Plant Biol. 67, 107–129 (2016). - PubMed
-
- López-Calcagno P. E., et al. , Stimulating photosynthetic processes increases productivity and water-use efficiency in the field. Nat. Plants 6, 1054–1063 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

