Cyclic Fasting-Mimicking Diet Plus Bortezomib and Rituximab Is an Effective Treatment for Chronic Lymphocytic Leukemia
- PMID: 38241703
- PMCID: PMC10982641
- DOI: 10.1158/0008-5472.CAN-23-0295
Cyclic Fasting-Mimicking Diet Plus Bortezomib and Rituximab Is an Effective Treatment for Chronic Lymphocytic Leukemia
Abstract
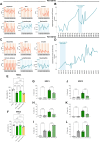
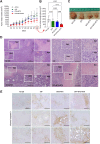
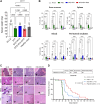
Cyclic fasting-mimicking diet (FMD) is an experimental nutritional intervention with potent antitumor activity in preclinical models of solid malignancies. FMD cycles are also safe and active metabolically and immunologically in cancer patients. Here, we reported on the outcome of FMD cycles in two patients with chronic lymphocytic leukemia (CLL) and investigated the effects of fasting and FMD cycles in preclinical CLL models. Fasting-mimicking conditions in murine CLL models had mild cytotoxic effects, which resulted in apoptosis activation mediated in part by lowered insulin and IGF1 concentrations. In CLL cells, fasting conditions promoted an increase in proteasome activity that served as a starvation escape pathway. Pharmacologic inhibition of this escape mechanism with the proteasome inhibitor bortezomib resulted in a strong enhancement of the proapoptotic effects of starvation conditions in vitro. In mouse CLL models, combining cyclic fasting/FMD with bortezomib and rituximab, an anti-CD20 antibody, delayed CLL progression and resulted in significant prolongation of mouse survival. Overall, the effect of proteasome inhibition in combination with FMD cycles in promoting CLL death supports the targeting of starvation escape pathways as an effective treatment strategy that should be tested in clinical trials.
Significance: Chronic lymphocytic leukemia cells resist fasting-mimicking diet by inducing proteasome activation to escape starvation, which can be targeted using proteasome inhibition by bortezomib treatment to impede leukemia progression and prolong survival.
©2024 The Authors; Published by the American Association for Cancer Research.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

