Therapeutic effects of long-term HBOT on Alzheimer's disease neuropathologies and cognitive impairment in APPswe/PS1dE9 mice
- PMID: 38241837
- PMCID: PMC10831255
- DOI: 10.1016/j.redox.2023.103006
Therapeutic effects of long-term HBOT on Alzheimer's disease neuropathologies and cognitive impairment in APPswe/PS1dE9 mice
Abstract
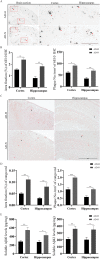
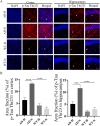
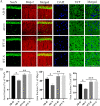
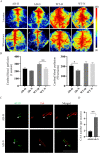
Alzheimer's disease (AD) is the most common neurodegenerative disorder with the pathological hallmarks of amyloid beta (Aβ) plaques and neurofibrillary tangles (NFTs) in the brain. Although there is a hope that anti-amyloid monoclonal antibodies may emerge as a new therapy for AD, the high cost and side effect is a big concern. Non-drug therapy is attracting more attention and may provide a better resolution for the treatment of AD. Given the fact that hypoxia contributes to the pathogenesis of AD, hyperbaric oxygen therapy (HBOT) may be an effective intervention that can alleviate hypoxia and improve AD. However, it remains unclear whether long-term HBOT intervention in the early stage of AD can slow AD progression and ultimately prevent cognitive impairment in this disease. In this study we applied consecutive 3-month HBOT interventions on 3-month-old APPswe/PS1dE9 AD mice which represent the early stage of AD. When the APPswe/PS1dE9 mice at 9-month-old which represent the disease stage we measured cognitive function, 24-h blood oxygen saturation, Aβ and tau pathologies, vascular structure and function, and neuroinflammation in APPswe/PS1dE9 mice. Our results showed that long-term HBOT can attenuate the impairments in cognitive function observed in 9-month-old APPswe/PS1dE9 mice. Most importantly, HBOT effectively reduced the progression of Aβ plaques deposition, hyperphosphorylated tau protein aggregation, and neuronal and synaptic degeneration in the AD mice. Further, long-term HBOT was able to enhance blood oxygen saturation level. Besides, long-term HBOT can improve vascular structure and function, and reduce neuroinflammation in AD mice. This study is the first to demonstrate that long-term HBOT intervention in the early stage of AD can attenuate cognitive impairment and AD-like pathologies. Overall, these findings highlight the potential of long-term HBOT as a disease-modifying approach for AD treatment.
Keywords: Alzheimer's disease; Amyloid plaques deposition; Blood oxygen saturation; Long-term hyperbaric oxygen therapy; Neuroinflammatory.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest Authors declare that there are no conflicts of interest.
Figures








Similar articles
-
Terahertz Irradiation Improves Cognitive Impairments and Attenuates Alzheimer's Neuropathology in the APPSWE/PS1DE9 Mouse: A Novel Therapeutic Intervention for Alzheimer's Disease.Neurosci Bull. 2024 Jul;40(7):857-871. doi: 10.1007/s12264-023-01145-3. Epub 2023 Nov 16. Neurosci Bull. 2024. PMID: 37971654 Free PMC article.
-
Differentiated Embryonic Neurospheres from Familial Alzheimer's Disease Model Show Innate Immune and Glial Cell Responses.Stem Cell Rev Rep. 2023 Aug;19(6):1800-1811. doi: 10.1007/s12015-023-10542-0. Epub 2023 May 2. Stem Cell Rev Rep. 2023. PMID: 37129730
-
Telmisartan Alleviates Alzheimer's Disease-Related Neuropathologies and Cognitive Impairments.J Alzheimers Dis. 2023;94(3):919-933. doi: 10.3233/JAD-230133. J Alzheimers Dis. 2023. PMID: 37355897
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Oxygen metabolism abnormality and Alzheimer's disease: An update.Redox Biol. 2023 Dec;68:102955. doi: 10.1016/j.redox.2023.102955. Epub 2023 Nov 8. Redox Biol. 2023. PMID: 37956598 Free PMC article. Review.
Cited by
-
Utilizing Hyperbaric Oxygen Therapy to Improve Cognitive Function in Patients With Alzheimer's Disease by Activating Autophagy-Related Signaling Pathways.Physiol Res. 2025 Mar 24;74(1):141-147. doi: 10.33549/physiolres.935447. Physiol Res. 2025. PMID: 40126150 Free PMC article.
-
Detrimental Roles of Hypoxia-Inducible Factor-1α in Severe Hypoxic Brain Diseases.Int J Mol Sci. 2024 Apr 18;25(8):4465. doi: 10.3390/ijms25084465. Int J Mol Sci. 2024. PMID: 38674050 Free PMC article. Review.
References
-
- Jutkowitz E., Pizzi L.T., Shewmaker P., Alarid-Escudero F., Epstein-Lubow G., Prioli K.M., Gaugler J.E., Gitlin L.N. Cost effectiveness of non-drug interventions that reduce nursing home admissions for people living with dementia. Alzheimers Dement. 2023;19(9):3867–3893. doi: 10.1002/alz.12964. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

