Targeting tumor-associated macrophages: Novel insights into immunotherapy of skin cancer
- PMID: 38242529
- PMCID: PMC11725115
- DOI: 10.1016/j.jare.2024.01.013
Targeting tumor-associated macrophages: Novel insights into immunotherapy of skin cancer
Abstract
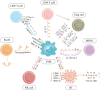
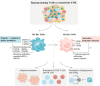
Background: The incidence of skin cancer is currently increasing, and conventional treatment options inadequately address the demands of disease management. Fortunately, the recent rapid advancement of immunotherapy, particularly immune checkpoint inhibitors (ICIs), has ushered in a new era for numerous cancer patients. However, the efficacy of immunotherapy remains suboptimal due to the impact of the tumor microenvironment (TME). Tumor-associated macrophages (TAMs), a major component of the TME, play crucial roles in tumor invasion, metastasis, angiogenesis, and immune evasion, significantly impacting tumor development. Consequently, TAMs have gained considerable attention in recent years, and their roles have been extensively studied in various tumors. However, the specific roles of TAMs and their regulatory mechanisms in skin cancer remain unclear.
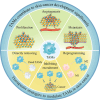
Aim of review: This paper aims to elucidate the origin and classification of TAMs, investigate the interactions between TAMs and various immune cells, comprehensively understand the precise mechanisms by which TAMs contribute to the pathogenesis of different types of skin cancer, and finally discuss current strategies for targeting TAMs in the treatment of skin cancer.
Key scientific concepts of overview: With a specific emphasis on the interrelationship between TAMs and skin cancer, this paper posits that therapeutic modalities centered on TAMs hold promise in augmenting and harmonizing with prevailing clinical interventions for skin cancer, thereby charting a novel trajectory for advancing the landscape of immunotherapeutic approaches for skin cancer.
Keywords: Immune checkpoint inhibitors; Immunotherapy; Skin cancer; Tumor-associated macrophages.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Kocarnik J.M., Compton K., Dean F.E., Fu W., Gaw B.L., Harvey J.D., et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: A systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2022;8:420–444. doi: 10.1001/jamaoncol.2021.6987. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

