Neurodevelopmental and synaptic defects in DNAJC6 parkinsonism, amenable to gene therapy
- PMID: 38242634
- PMCID: PMC11146427
- DOI: 10.1093/brain/awae020
Neurodevelopmental and synaptic defects in DNAJC6 parkinsonism, amenable to gene therapy
Abstract
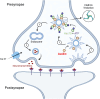
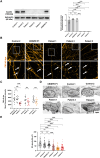
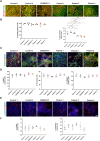
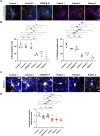
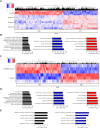
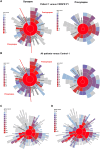
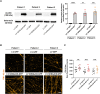
DNAJC6 encodes auxilin, a co-chaperone protein involved in clathrin-mediated endocytosis (CME) at the presynaptic terminal. Biallelic mutations in DNAJC6 cause a complex, early-onset neurodegenerative disorder characterized by rapidly progressive parkinsonism-dystonia in childhood. The disease is commonly associated with additional neurodevelopmental, neurological and neuropsychiatric features. Currently, there are no disease-modifying treatments for this condition, resulting in significant morbidity and risk of premature mortality. To investigate the underlying disease mechanisms in childhood-onset DNAJC6 parkinsonism, we generated induced pluripotent stem cells (iPSC) from three patients harbouring pathogenic loss-of-function DNAJC6 mutations and subsequently developed a midbrain dopaminergic neuronal model of disease. When compared to age-matched and CRISPR-corrected isogenic controls, the neuronal cell model revealed disease-specific auxilin deficiency as well as disturbance of synaptic vesicle recycling and homeostasis. We also observed neurodevelopmental dysregulation affecting ventral midbrain patterning and neuronal maturation. To explore the feasibility of a viral vector-mediated gene therapy approach, iPSC-derived neuronal cultures were treated with lentiviral DNAJC6 gene transfer, which restored auxilin expression and rescued CME. Our patient-derived neuronal model provides deeper insights into the molecular mechanisms of auxilin deficiency as well as a robust platform for the development of targeted precision therapy approaches.
Keywords: CME; DNAJC6; auxilin; gene therapy; neurodevelopmental; parkinsonism.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures
References
-
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. - PubMed
-
- Köroĝlu Ç, Baysal L, Cetinkaya M, Karasoy H, Tolun A. DNAJC6 is responsible for juvenile parkinsonism with phenotypic variability. Park Relat Disord. 2013;19:320–324. - PubMed
-
- Olgiati S, Quadri M, Fang M, et al. DNAJC6 mutations associated with early-onset Parkinson’s disease. Ann Neurol. 2015;79:244–256. - PubMed
-
- Elsayed LEO, Drouet V, Usenko T, et al. A novel nonsense mutation in DNAJC6 expands the phenotype of autosomal-recessive juvenile-onset Parkinson’s disease. Ann Neurol. 2016;79:335–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

