Ipilimumab and nivolumab combined with anthracycline-based chemotherapy in metastatic hormone receptor-positive breast cancer: a randomized phase 2b trial
- PMID: 38242720
- PMCID: PMC10806573
- DOI: 10.1136/jitc-2023-007990
Ipilimumab and nivolumab combined with anthracycline-based chemotherapy in metastatic hormone receptor-positive breast cancer: a randomized phase 2b trial
Abstract
Background: Immune checkpoint inhibitors have shown minimal clinical activity in hormone receptor-positive metastatic breast cancer (HR+mBC). Doxorubicin and low-dose cyclophosphamide are reported to induce immune responses and counter regulatory T cells (Tregs). Here, we report the efficacy and safety of combined programmed cell death protein-1/cytotoxic T-lymphocyte-associated protein 4 blockade concomitant with or after immunomodulatory chemotherapy for HR+mBC.
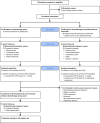
Methods: Patients with HR+mBC starting first-/second- line chemotherapy (chemo) were randomized 2:3 to chemotherapy (pegylated liposomal doxorubicin 20 mg/m2 every second week plus cyclophosphamide 50 mg by mouth/day in every other 2-week cycle) with or without concomitant ipilimumab (ipi; 1 mg/kg every sixth week) and nivolumab (nivo; 240 mg every second week). Patients in the chemo-only arm were offered cross-over to ipi/nivo without chemotherapy. Co-primary endpoints were safety in all patients starting therapy and progression-free survival (PFS) in the per-protocol (PP) population, defined as all patients evaluated for response and receiving at least two treatment cycles. Secondary endpoints included objective response rate, clinical benefit rate, Treg changes during therapy and assessment of programmed death-ligand 1 (PD-L1), mutational burden and immune gene signatures as biomarkers.
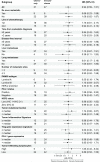
Results: Eighty-two patients were randomized and received immune-chemo (N=49) or chemo-only (N=33), 16 patients continued to the ipi/nivo-only cross-over arm. Median follow-up was 41.4 months. Serious adverse events occurred in 63% in the immune-chemo arm, 39% in the chemo-only arm and 31% in the cross-over-arm. In the PP population (N=78) median PFS in the immune-chemo arm was 5.1 months, compared with 3.6 months in the chemo-only arm, with HR 0.94 (95% CI 0.59 to 1.51). Clinical benefit rates were 55% (26/47) and 48% (15/31) in the immune-chemo and chemo-only arms, respectively. In the cross-over-arm (ipi/nivo-only), objective responses were observed in 19% of patients (3/16) and clinical benefit in 25% (4/16). Treg levels in blood decreased after study chemotherapy. High-grade immune-related adverse events were associated with prolonged PFS. PD-L1 status and mutational burden were not associated with ipi/nivo benefit, whereas a numerical PFS advantage was observed for patients with a high Treg gene signature in tumor.
Conclusion: The addition of ipi/nivo to chemotherapy increased toxicity without improving efficacy. Ipi/nivo administered sequentially to chemotherapy was tolerable and induced clinical responses.
Trial registration number: ClinicalTrials.gov Identifier: NCT03409198.
Keywords: Breast Neoplasms; Immune Checkpoint Inhibitors; Ipilimumab; Nivolumab; T-Lymphocytes, Regulatory.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JAK has in the last 5 years received research support from Bristol Myers Squibb, F. Hoffmann-La Roche, NanoString, and NEC OncoImmunity and has previously received advisory board/lecture honoraria from pharmaceutical companies, including Bristol Myers Squibb. CQ has received honoraria for advisory board from AstraZeneca. BG has received honoraria for advisory boards from Eli Lilly, Gilead, Daiichi Sankyo, Roche, and Pierre Fabre. LJ has received lecture honoraria from Pfizer, Novartis, and AstraZeneca. AG has received travel grants or honoraria for advisory boards from Lilly, Daiichi Sankyo, Seagen, Pfizer, and AstraZeneca. HGR has received research support from Illumina and NanoString. OCL has over the last 2 years received honoraria for work as statistical advisor for Novartis. All other authors declare no competing interests.
Figures


References
-
- Cortes J, Cescon DW, Rugo HS, et al. . Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020;396:1817–28. 10.1016/S0140-6736(20)32531-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
