Oxide nanolitisation-induced melt iron extraction causes viscosity jumps and enhanced explosivity in silicic magma
- PMID: 38242880
- PMCID: PMC10799068
- DOI: 10.1038/s41467-024-44850-x
Oxide nanolitisation-induced melt iron extraction causes viscosity jumps and enhanced explosivity in silicic magma
Abstract
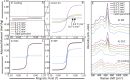
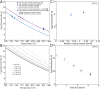
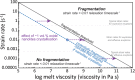
Explosivity in erupting volcanoes is controlled by the degassing dynamics and the viscosity of the ascending magma in the conduit. Magma crystallisation enhances both heterogeneous bubble nucleation and increases in magma bulk viscosity. Nanolite crystallisation has been suggested to enhance such processes too, but in a noticeably higher extent. Yet the precise causes of the resultant strong viscosity increase remain unclear. Here we report experimental results for rapid nanolite crystallisation in natural silicic magma and the extent of the subsequent viscosity increase. Nanolite-free and nanolite-bearing rhyolite magmas were subjected to heat treatments, where magmas crystallised or re-crystallised oxide nanolites depending on their initial state, showing an increase of one order of magnitude as oxide nanolites formed. We thus demonstrate that oxide nanolites crystallisation increases magma bulk viscosity mainly by increasing the viscosity of its melt phase due to the chemical extraction of iron, whereas the physical effect of particle suspension is minor, almost negligible. Importantly, we further observe that this increase is sufficient for driving magma fragmentation depending on magma degassing and ascent dynamics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Blundy J, Cashman K. Ascent-driven crystallisation of dacite magmas at Mount St Helens, 1980–1986. Contrib. Mineral. Petrol. 2001;140:631–650. doi: 10.1007/s004100000219. - DOI
-
- Hammer JE, Rutherford MJ. An experimental study of the kinetics of decompression-induced crystallization in silicic melt. J. Geophys. Res. 2002;107:B1. doi: 10.1029/2001JB000281. - DOI
-
- Pistone M, Formo E, Whittington AG, Herbst T, Cottrell E. Direct nanoscale observations of degassing‑induced crystallisation in felsic magmas. Contrib. Mineral. Petrol. 2022;177:38. doi: 10.1007/s00410-022-01900-1. - DOI
-
- Zhang Y, Xu Z, Zhu M, Wang H. Silicate melt properties and volcanic eruptions. Rev. Geophys. 2007;45:RG4004. doi: 10.1029/2006RG000216. - DOI
-
- Hui H, Zhang Y. Toward a general viscosity equation for natural anhydrous and hydrous silicate melts. Geochim. Cosmochim. Acta. 2007;71:403–416. doi: 10.1016/j.gca.2006.09.003. - DOI
LinkOut - more resources
Full Text Sources

