Brain-derived neurotrophic factor from microglia regulates neuronal development in the medial prefrontal cortex and its associated social behavior
- PMID: 38243072
- PMCID: PMC11189755
- DOI: 10.1038/s41380-024-02413-y
Brain-derived neurotrophic factor from microglia regulates neuronal development in the medial prefrontal cortex and its associated social behavior
Abstract
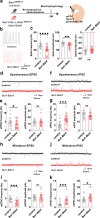
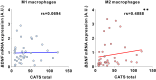
Microglia and brain-derived neurotrophic factor (BDNF) are essential for the neuroplasticity that characterizes critical developmental periods. The experience-dependent development of social behaviors-associated with the medial prefrontal cortex (mPFC)-has a critical period during the juvenile period in mice. However, whether microglia and BDNF affect social development remains unclear. Herein, we aimed to elucidate the effects of microglia-derived BDNF on social behaviors and mPFC development. Mice that underwent social isolation during p21-p35 had increased Bdnf in the microglia accompanied by reduced adulthood sociability. Additionally, transgenic mice overexpressing microglial Bdnf-regulated using doxycycline at different time points-underwent behavioral, electrophysiological, and gene expression analyses. In these mice, long-term overexpression of microglial BDNF impaired sociability and excessive mPFC inhibitory neuronal circuit activity. However, administering doxycycline to normalize BDNF from p21 normalized sociability and electrophysiological function in the mPFC, whereas normalizing BDNF from later ages (p45-p50) did not normalize electrophysiological abnormalities in the mPFC, despite the improved sociability. To evaluate the possible role of BDNF in human sociability, we analyzed the relationship between adverse childhood experiences and BDNF expression in human macrophages, a possible proxy for microglia. Results show that adverse childhood experiences positively correlated with BDNF expression in M2 but not M1 macrophages. In summary, our study demonstrated the influence of microglial BDNF on the development of experience-dependent social behaviors in mice, emphasizing its specific impact on the maturation of mPFC function, particularly during the juvenile period. Furthermore, our results propose a translational implication by suggesting a potential link between BDNF secretion from macrophages and childhood experiences in humans.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Update of
-
Brain-derived neurotrophic factor from microglia regulates neuronal development in the medial prefrontal cortex and its associated social behavior.Res Sq [Preprint]. 2023 Jun 30:rs.3.rs-3094335. doi: 10.21203/rs.3.rs-3094335/v1. Res Sq. 2023. Update in: Mol Psychiatry. 2024 May;29(5):1338-1349. doi: 10.1038/s41380-024-02413-y. PMID: 37461488 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- R03 HD108551/HD/NICHD NIH HHS/United States
- R01 DC015776/DC/NIDCD NIH HHS/United States
- R01 MH099660/MH/NIMH NIH HHS/United States
- R21 HD105287/HD/NICHD NIH HHS/United States
- R21 HD053114/HD/NICHD NIH HHS/United States
- 21gm6310015h0002/Japan Agency for Medical Research and Development (AMED)
- 23gm1510009h0002/Japan Agency for Medical Research and Development (AMED)
- 21wm04250XXs0101/Japan Agency for Medical Research and Development (AMED)
- 23gm1910004s0301/Japan Agency for Medical Research and Development (AMED)
- 20K16674/MEXT | Japan Society for the Promotion of Science (JSPS)
- R01MH099660/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01DC015776/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R21HD053114/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R03HD108551/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R21HD105287/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

