An ETFDH-driven metabolon supports OXPHOS efficiency in skeletal muscle by regulating coenzyme Q homeostasis
- PMID: 38243131
- PMCID: PMC10896730
- DOI: 10.1038/s42255-023-00956-y
An ETFDH-driven metabolon supports OXPHOS efficiency in skeletal muscle by regulating coenzyme Q homeostasis
Abstract
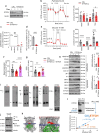
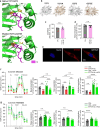
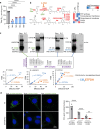
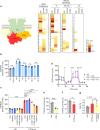
Coenzyme Q (Q) is a key lipid electron transporter, but several aspects of its biosynthesis and redox homeostasis remain undefined. Various flavoproteins reduce ubiquinone (oxidized form of Q) to ubiquinol (QH2); however, in eukaryotes, only oxidative phosphorylation (OXPHOS) complex III (CIII) oxidizes QH2 to Q. The mechanism of action of CIII is still debated. Herein, we show that the Q reductase electron-transfer flavoprotein dehydrogenase (ETFDH) is essential for CIII activity in skeletal muscle. We identify a complex (comprising ETFDH, CIII and the Q-biosynthesis regulator COQ2) that directs electrons from lipid substrates to the respiratory chain, thereby reducing electron leaks and reactive oxygen species production. This metabolon maintains total Q levels, minimizes QH2-reductive stress and improves OXPHOS efficiency. Muscle-specific Etfdh-/- mice develop myopathy due to CIII dysfunction, indicating that ETFDH is a required OXPHOS component and a potential therapeutic target for mitochondrial redox medicine.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

